Preparation method of 2-methyl-3-methoxybenzoic acid
A technology of methoxybenzoic acid and hydroxybenzoic acid, which is applied in the field of compound preparation, can solve the problems of long reaction time, increased energy consumption, and high risk factor, and achieves the effects of determining the feasibility of the solution, simplifying the production process, and reducing the production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] The present embodiment provides a method for preparing 2-methyl-3-hydroxybenzoic acid.
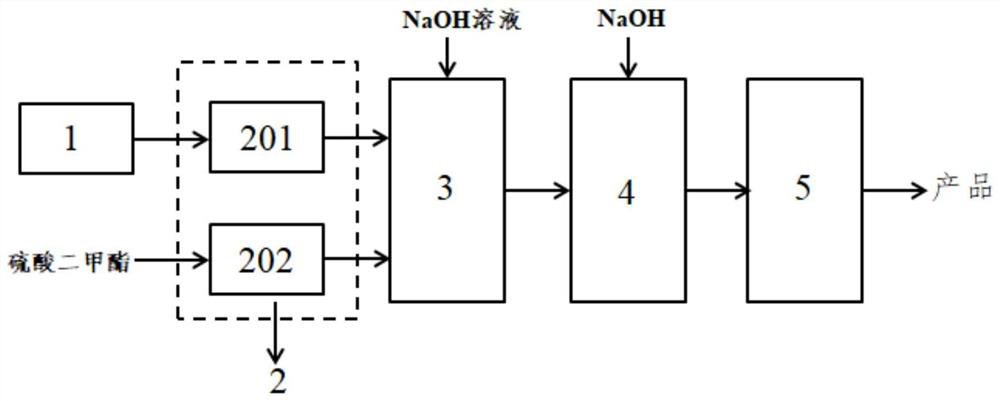
[0078] (1) Raw material 2-methyl-3-hydroxybenzoic acid and 20% sodium hydroxide solution was prepared in a compound kettle in accordance with the molar ratio of 2-methyl-3-hydroxybenzoic acid and sodium hydroxide of 1:2.5 to obtain a sodium phenolic solution;
[0079] (2) The sodium phenolic solution is transported by the first feed pump at a flow rate of 18.17kg / min to the microchannel reactor with a holding liquid capacity of 1L and the dimethyl sulfate conveyed by the second feed pump is reacted in contact with the microchannel reactor at a temperature of 50 ° C to form an alkylation reaction liquid, wherein the molar ratio of raw materials to dimethyl sulfate is 1:2.4, the alkylation reaction solution stays in the microchannel reactor for 3 seconds and then transfers to the aging reactor, while the same molar amount of 20% sodium hydroxide solution with the raw material is 4.57kg / T...
Embodiment 2
[0083] The present embodiment provides a method for preparing 2-methyl-3-hydroxybenzoic acid.
[0084] (1) The raw material 2-methyl-3-hydroxybenzoic acid is added to the ingredient kettle, and the water is stirred to add 30% sodium hydroxide solution to form a sodium phenolic solution, of which the molar ratio of raw material to sodium hydroxide is 1:4, and the mass ratio of sodium hydroxide to water is 20:100;
[0085] (2) The sodium phenolic solution is transported by the first feed pump at a flow rate of 26.53kg / min to the microchannel reactor with a holding liquid capacity of 5L and the dimethyl sulfate transported by the second feed pump is in contact with the reaction at a temperature of 55 ° C in the microchannel reactor to form an alkylation reaction liquid, wherein the molar ratio of raw materials to dimethyl sulfate is 1:2.5, and the alkylation reaction liquid stays in the microchannel reactor for 10 seconds and then transfers to the aging reactor, and is further aged f...
Embodiment 3
[0089] The present embodiment provides a method for preparing 2-methyl-3-hydroxybenzoic acid.
[0090] (1) Raw material 2-methyl-3-hydroxybenzoic acid and 20% sodium hydroxide solution in accordance with the molar ratio of 2-methyl 3-hydroxybenzoic acid and sodium hydroxide is 1:5 to form a sodium phenol solution;
[0091] (2) The sodium phenolic solution is transported by the first feed pump at a flow rate of 32.1kg / min to the microchannel reactor with a liquid holding capacity of 40L and the dimethyl sulfate transported by the second feed pump is reacted in contact with the microchannel reactor at a temperature of 60 ° C to form an alkylation reaction solution, wherein the molar ratio of raw materials to dimethyl sulfate is 1:2.2, and the alkylation reaction solution stays in the microchannel reactor for 120s;
[0092] (3) The alkyl reaction solution flows into the hydrolysis reaction unit of the hydrolysis reaction unit through the pipeline, and the 30% sodium hydroxide solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com