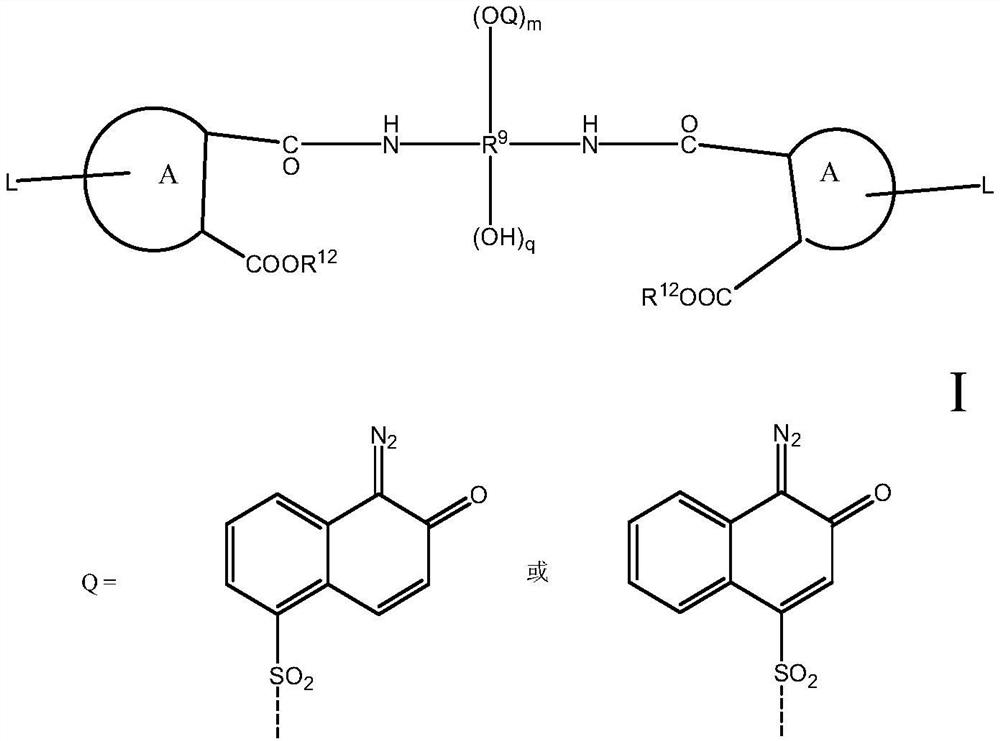
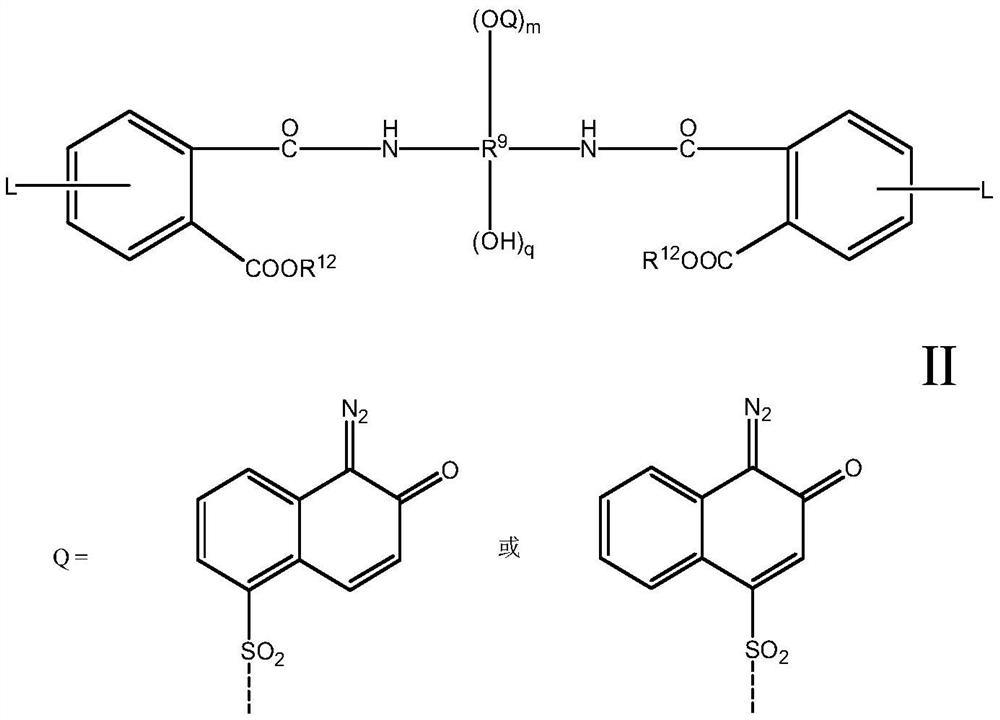
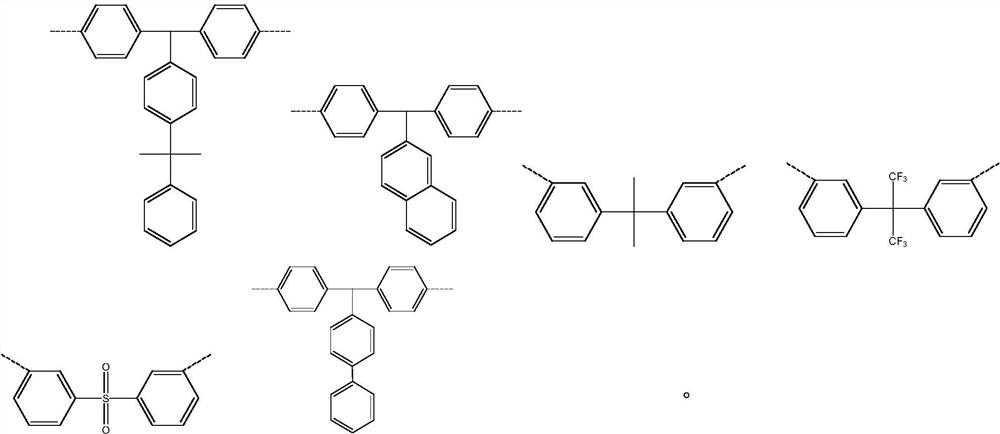
Diazanaphthoquinone sulfonate compound and resin composition
A technology of naphthoquinone sulfonate and compound is applied in the field of photolithography technology and can solve problems such as unfavorable long-term stability of devices and incomplete reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0075] The resins, compounds and compositions of the present invention were synthesized representatively, and the properties of each resin composition were tested.
[0076] The following synthesis examples of the present invention exemplarily provide specific synthetic methods of representative compounds. Solvents and reagents used in the following synthesis examples can be purchased or customized from the domestic chemical product market, such as purchased from Sinopharm Group Reagent Company, Sigma-AldRich Company, Bailingwei Reagent Co., Ltd., etc.
preparation example 1
[0078] Synthesis of diamine intermediate 1:
[0079] Synthetic intermediate 4-(2-(4-hydroxyphenyl)propan-2-yl)benzene (form)aldehyde:
[0080] Argon atmosphere, POCl at -5°C 3 (11.2 mL, 0.12 mol) was slowly added dropwise into 60 mL of DMF. Add the DMF solution of 4-cinnamon phenol dropwise into the reaction system, slowly raise the temperature of the reaction system to 60°C, and keep it warm for 3 hours. After the temperature of the reaction system drops to room temperature, pour the reaction mixture into ice cubes, and the system After neutralization to neutrality, extract with ethyl acetate, collect the organic phase, wash with saturated sodium chloride and deionized water, and remove most of the solvent by rotary evaporation. After vacuum drying at 50°C, pass the crude product of the target product through the column layer The target product was purified by analytical method.
[0081]
[0082] ZAB-HS mass spectrometer (manufactured by Micromass, UK), M / Z (M+1): 241. ...
preparation example 2
[0087] Synthesis of diamine intermediate 2:
[0088] Under nitrogen protection, weigh 18.62g (0.1mol) 6-methoxy-2-naphthaldehyde in a 100mL three-necked flask, weigh 36.9g (0.3mol) o-methoxyaniline and add it to the three-necked flask, and heat up to React at 150°C for 3 hours, recrystallize the obtained crude product with ethanol, azeotrope with hydroiodic acid for 2 hours under nitrogen protection, then add sodium bicarbonate to neutralize hydroiodic acid, collect the precipitate, and obtain diamine intermediate 2.
[0089]
[0090] ZAB-HS mass spectrometer (manufactured by Micromass, UK), M / Z (M+1): 373.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com