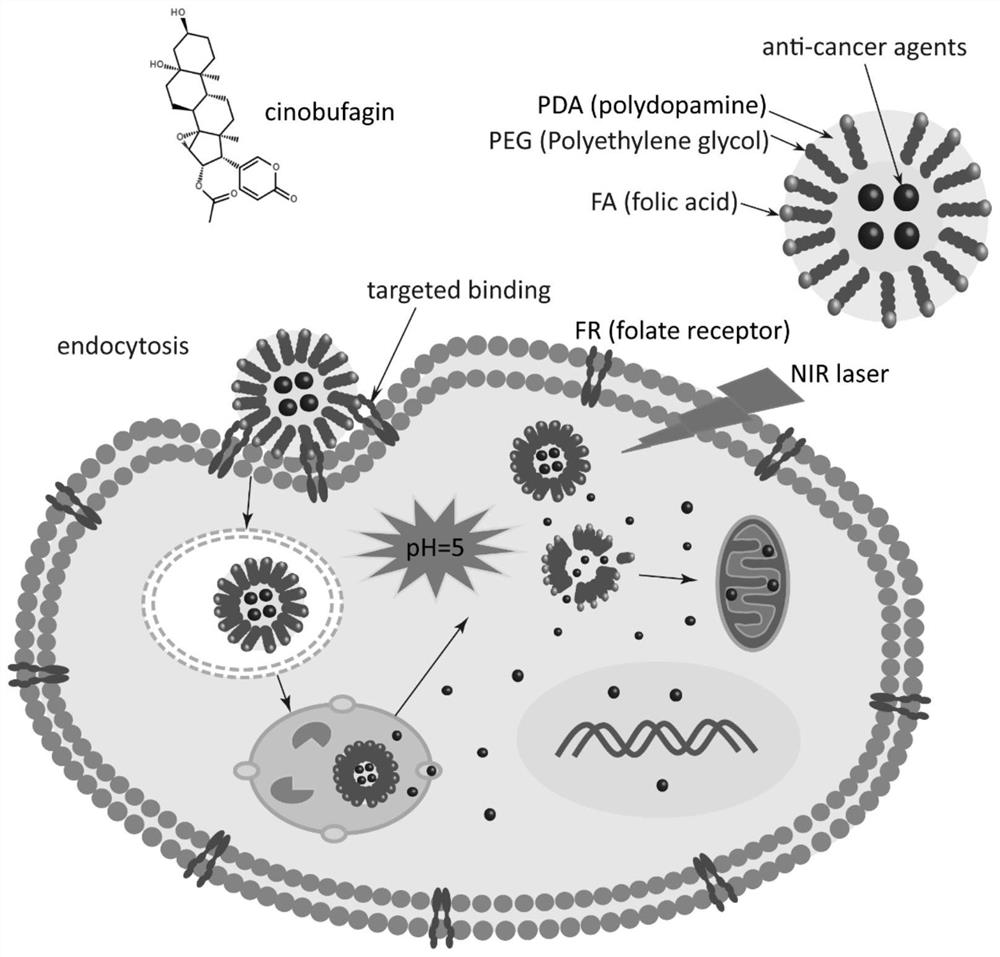
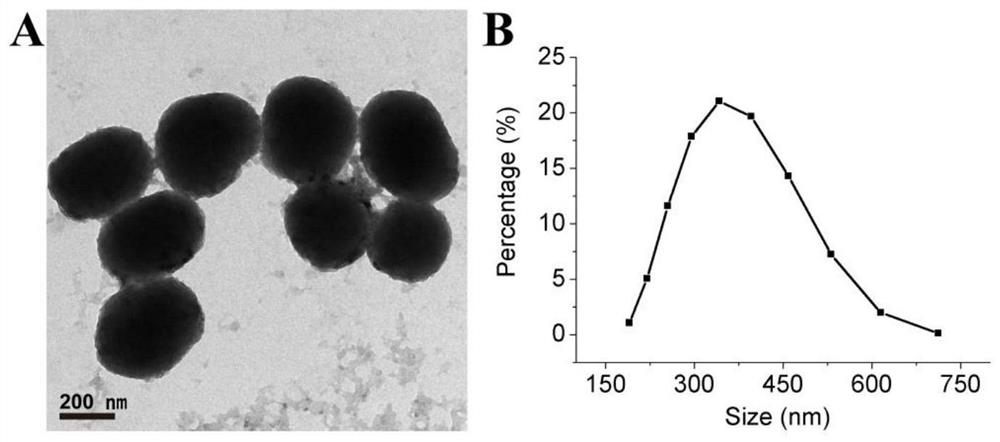
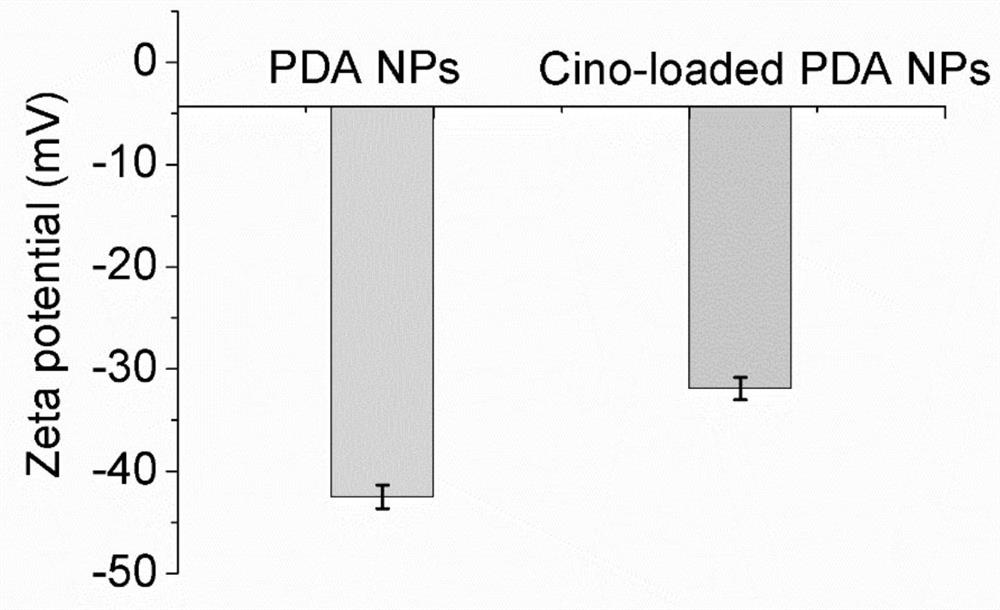
Cinobufagin-loaded PDA nano-drug as well as preparation method and application thereof
A nano-drug, cinobufacin technology, applied in nano-drugs, drug combinations, nanotechnology, etc., can solve problems such as short circulation half-life, low bioavailability, poor water solubility of cinobufacin, and achieve good biocompatibility, The effect of uniform distribution, good colloidal stability and biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1--Preparation method of ammonia water for PDA nanomedicine loaded with cinobufacini
[0030] (1) First, the blank PDA nanocarriers or the PDA nanomedicines loaded with cinobufagin were synthesized by using ammonia water in alcohol solution. First mix 290mL ultrapure water, 110mL ethanol and 1.5mL NH 4 OH was stirred at room temperature for 30 minutes. Then 50 mg of cinobufacini or 50 mg of doxorubicin (no blank nanocarrier added) was added to 10 ml of ethanol. Add 0.5 g of dopamine hydrochloride to 10 mL of ultrapure water, and stir overnight. Finally, the nano-drugs were collected by centrifugation, centrifuged at 10,000 rmp for 10 minutes, the unloaded drug was taken out, centrifuged at room temperature, washed twice with ultrapure water, and dried on a freeze dryer overnight.
[0031] (2) FA was then modified on the surface of nanoparticles with EDC\NHS. First, the above nanodrugs (100 mg) were dispersed in 10 ml of PBS buffer (10 mM, pH=6.0). Subsequen...
Embodiment 2
[0032] Embodiment 2--Tris-HCl preparation method of PDA nanomedicine loaded with cinobufacini
[0033] First, 72 mL of ethanol was added to 400 mL of Tris-HCl (10 mM, pH=8.5) buffer solution, and the mixture was stirred uniformly at 37°C. Then 50 mg of cinobufacini or 50 mg of doxorubicin (without the blank nanocarrier) was added to 10 ml of ethanol. Add 0.5 g of dopamine hydrochloride to 10 mL of ultrapure water, and stir overnight. During the reaction, the mixed solution rapidly changed from colorless to black, indicating the formation of PDA nanocarriers. Finally, the nano-drugs were collected by centrifugation, centrifuged at 10,000 rmp for 10 minutes, the supernatant was discarded, the unloaded drug was removed, centrifuged at room temperature, washed twice with ultrapure water, and dried on a freeze dryer overnight.
[0034] For the modification of targeting molecule FA, the above nanodrugs (100 mg) were first dispersed in 10 ml of PBS buffer (10 mM, pH=6.0). Subseque...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com