Glycopeptide-enriched polymer material as well as preparation and application thereof
A technology of polymer materials and polymerization reactions, which is applied in the fields of material analysis chemistry and organic chemistry, can solve the problems of inability to enrich glycopeptides, non-specific adsorption of acidic peptide chains with basic residues of glycopeptides that are difficult to retain, and glycopeptides that are difficult to wash. To achieve the effect of controllable polymer layer, high repeatability and enrichment, and easy repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of glycopeptide enriched materials
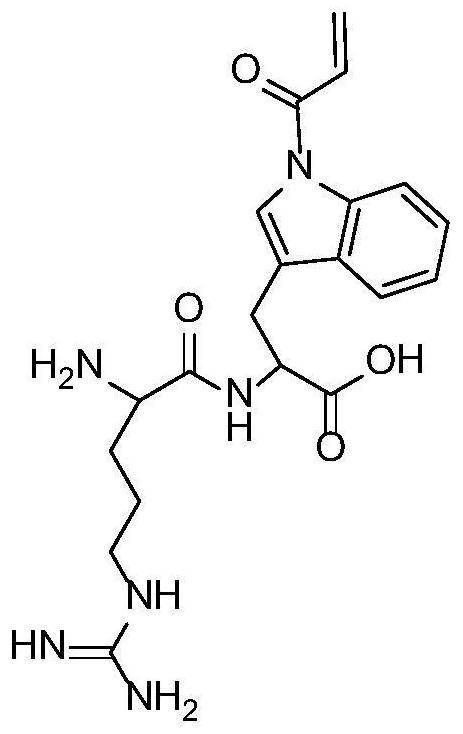
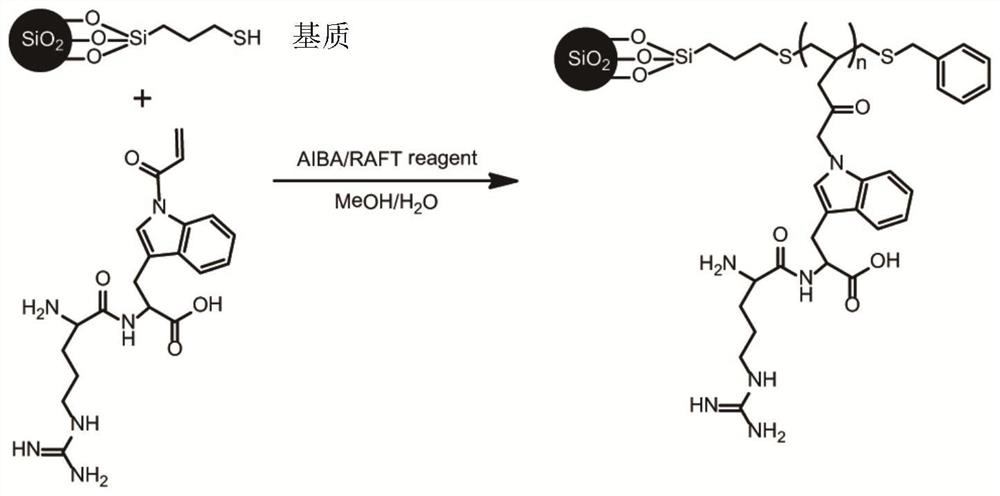
[0044] The dipeptide functional monomer structure composed of tryptophan and arginine is as follows figure 1 shown. Into a 50ml three-necked flask were sequentially added 0.5mmol tryptophan-arginine dipeptide monomer, 0.005mmol chain transfer reagent 4-cyanovaleric acid dithiobenzoic acid, 0.001mmol initiator azobisisobutyramidine hydrochloride, the molar ratio is 500:5:1, and then 0.5 g of mercapto silica gel (particle size 5 μm, pore size is added) ), while adding 6 mL of ultrapure water and 6 mL of methanol as solvent. Nitrogen was introduced under stirring, and after thorough mixing, the reaction system was evacuated and filled with nitrogen to remove residual oxygen in the reaction system; the temperature of the flask was controlled at 70 ° C and left to react for 48 hours; Ionized water (H 2 O) Sequentially washing the polymer grafted surface to obtain glycopeptide-enriched material, and drying in an oven.
[...
Embodiment 2
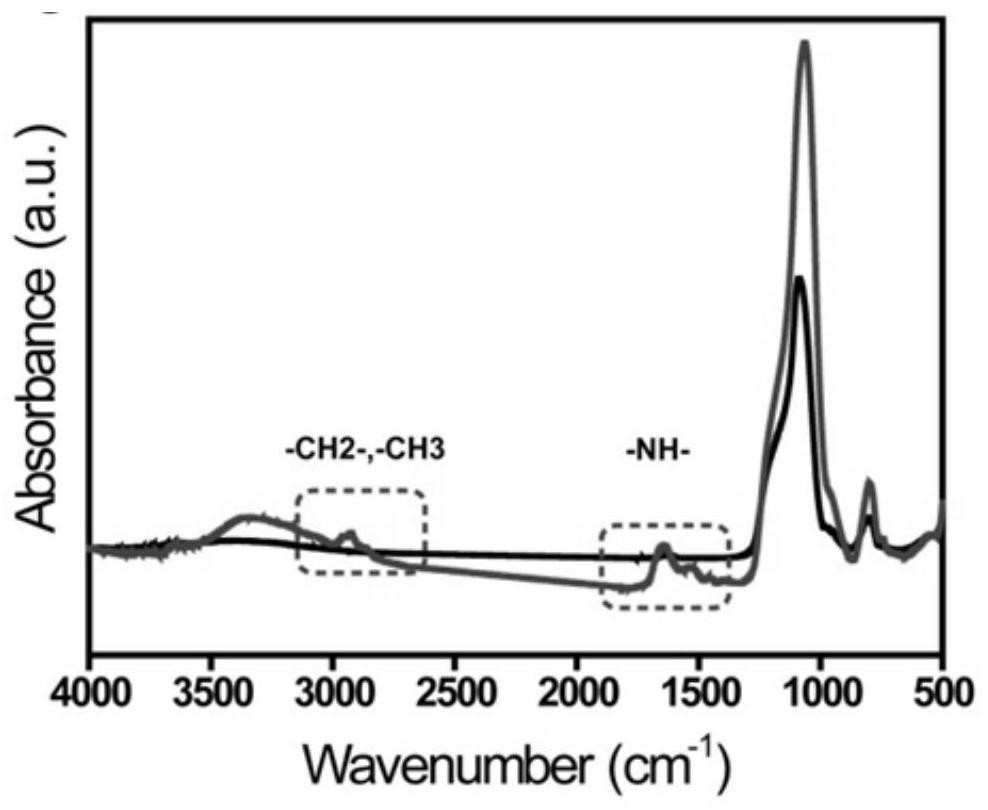
[0047] The dipeptide monomer copolymer composed of arginine and tryptophan was grafted onto the surface of porous silica gel as described in Example 1, and an infrared characterization experiment was carried out. image 3 The infrared absorption spectrum of the copolymer modified material is shown, indicating that the dipeptide monomer has been successfully bonded to the surface of the porous silica gel. Of which, ~1640cm -1 The wave peak at is attributed to the absorption peak of C=O; ~2900cm -1 The peaks at are assigned to the methyl peak and the methylene peak.
Embodiment 3
[0049] As described in Example 1, the dipeptide monomer composed of arginine and tryptophan and the sialic acid monosaccharide molecule were subjected to isothermal titration experiments. Figure 4 It is shown that there is an interaction between the dipeptide molecule and the sialic acid monosaccharide molecule, and the two generate exothermic heat after binding, and the binding is carried out in a 1:2 binding manner, and the relevant binding coefficient K is shown in the figure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com