Method for preparing sodium carbonate and co-producing ammonium sulfate by using sodium sulfate
A technology of sodium sulfate and sodium carbonate, applied in carbonate preparations, chemical instruments and methods, products, etc., can solve the problems of cumbersome control, increase of calcium residues, reactor scarring, etc., to save the ammonia distillation process, simplify the process Process flow, the effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
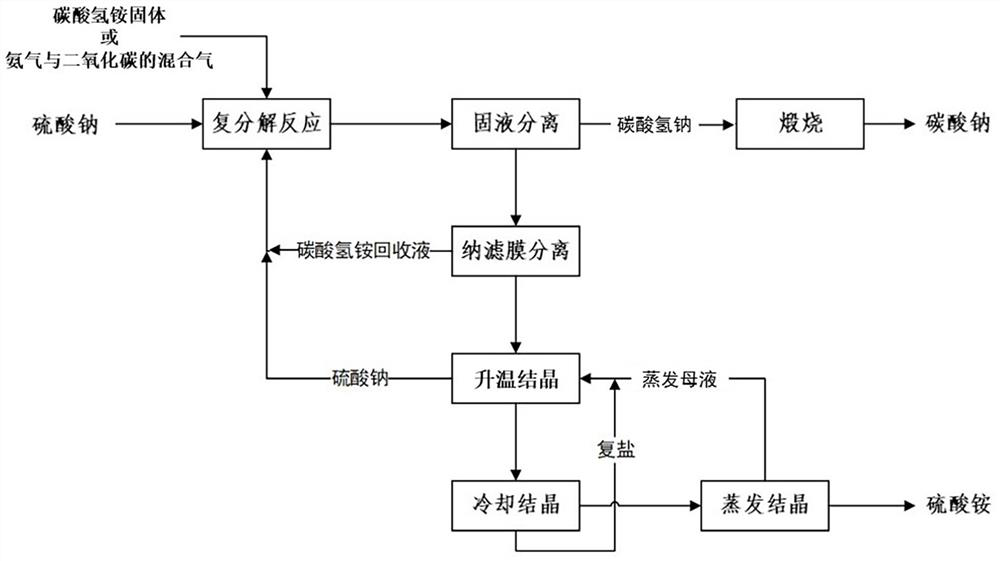
[0047] The present embodiment provides a method for utilizing sodium sulfate to prepare sodium carbonate and co-producing ammonium sulfate, such as figure 1 As shown, the method includes the following steps:
[0048] (1) Mix the mixture of sodium sulfate, ammonium bicarbonate recovery liquid, ammonia gas and carbon dioxide, and control the molar ratio of total ammonium to total sodium in the reaction system to be 1.1:1, and carry out the metathesis reaction at 35 °C, and the reaction time is 1h, first settling separation and then filtration separation to obtain sodium bicarbonate and metathesis mother liquor; the obtained sodium bicarbonate is calcined to obtain sodium carbonate;
[0049] (2) separating the metathesis mother liquor obtained in step (1) through a nanofiltration membrane at 35°C to obtain an ammonium bicarbonate recovery solution and a sulfate concentrate; the obtained ammonium bicarbonate recovery solution is reused in step (1);
[0050] (3) Mixed Na 2 SO 4 ...
Embodiment 2
[0055] The present embodiment provides a method for utilizing sodium sulfate to prepare sodium carbonate and co-producing ammonium sulfate, such as figure 1 As shown, the method includes the following steps:
[0056] (1) Mix sodium sulfate, ammonium bicarbonate recovery liquid, and ammonium bicarbonate solid, and control the molar ratio of total ammonium to total sodium in the reaction system to be 0.9:1, carry out metathesis reaction at 50 °C, and the reaction time is 0.5h, Sodium bicarbonate and metathesis mother liquor are obtained after first sedimentation separation and then filtration separation; the obtained sodium bicarbonate is calcined to obtain sodium carbonate;
[0057] (2) separating the metathesis mother liquor obtained in step (1) through a nanofiltration membrane at 60° C. to obtain an ammonium bicarbonate recovery solution and a sulfate concentrate; the obtained ammonium bicarbonate recovery solution is reused in step (1);
[0058] (3) Mixed Na 2 SO 4 ·(NH ...
Embodiment 3
[0063] The present embodiment provides a method for utilizing sodium sulfate to prepare sodium carbonate and co-producing ammonium sulfate, such as figure 1 As shown, the method includes the following steps:
[0064] (1) Mix sodium sulfate, ammonium bicarbonate recovery liquid, mixed gas of ammonia and carbon dioxide, and control the molar ratio of total ammonium to total sodium in the reaction system to be 1.4:1, and carry out metathesis reaction at 20 °C, and the reaction time is 3h, first settling separation and then filtration separation to obtain sodium bicarbonate and metathesis mother liquor; the obtained sodium bicarbonate is calcined to obtain sodium carbonate;
[0065] (2) separating the metathesis mother liquor obtained in step (1) through a nanofiltration membrane at 10° C. to obtain an ammonium bicarbonate recovery solution and a sulfate concentrate; the obtained ammonium bicarbonate recovery solution is reused in step (1);
[0066] (3) Mixed Na 2 SO 4 ·(NH 4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com