Transition metal phosphide with phosphorus vacancies filled with non-metallic elements, preparation of transition metal phosphide and lithium-sulfur battery
A technology of non-metallic elements and transition metals, which is applied in the field of preparation of inorganic compounds, can solve problems such as the reduction of sulfur utilization rate and coulombic efficiency, the poor conductivity of sulfur and discharge products, and the hindrance of electron and ion conduction, so as to improve the kinetics of chemical reactions And cycle stability, improve electron transport performance, improve the effect of catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
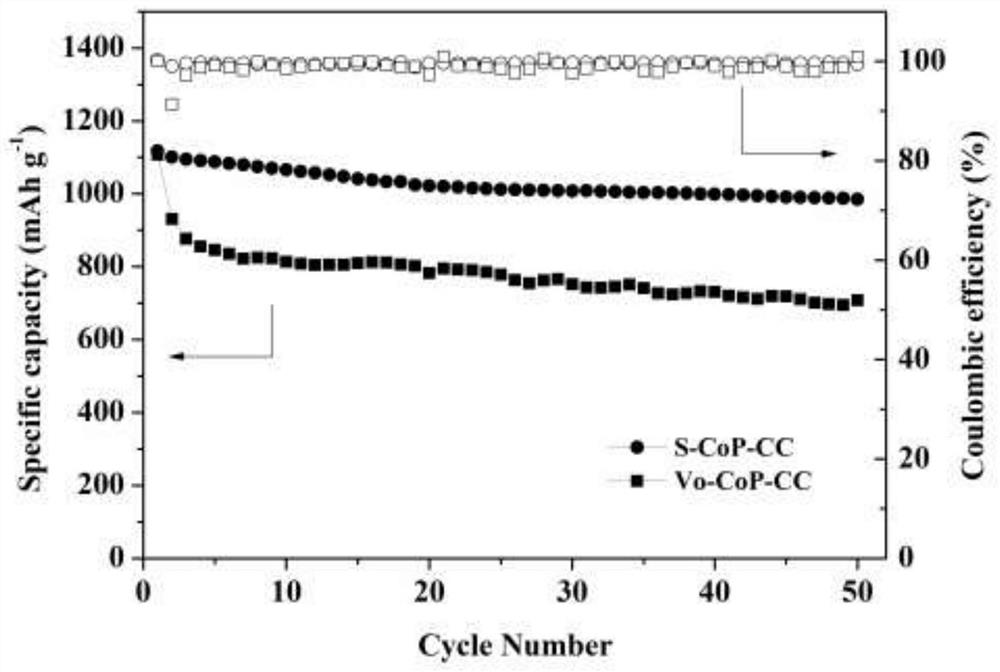
[0041] A preparation method of a cobalt phosphide electrode (abbreviated as: S-CoP-CC) filled with phosphorus vacancies with sulfur element, the method steps are as follows:
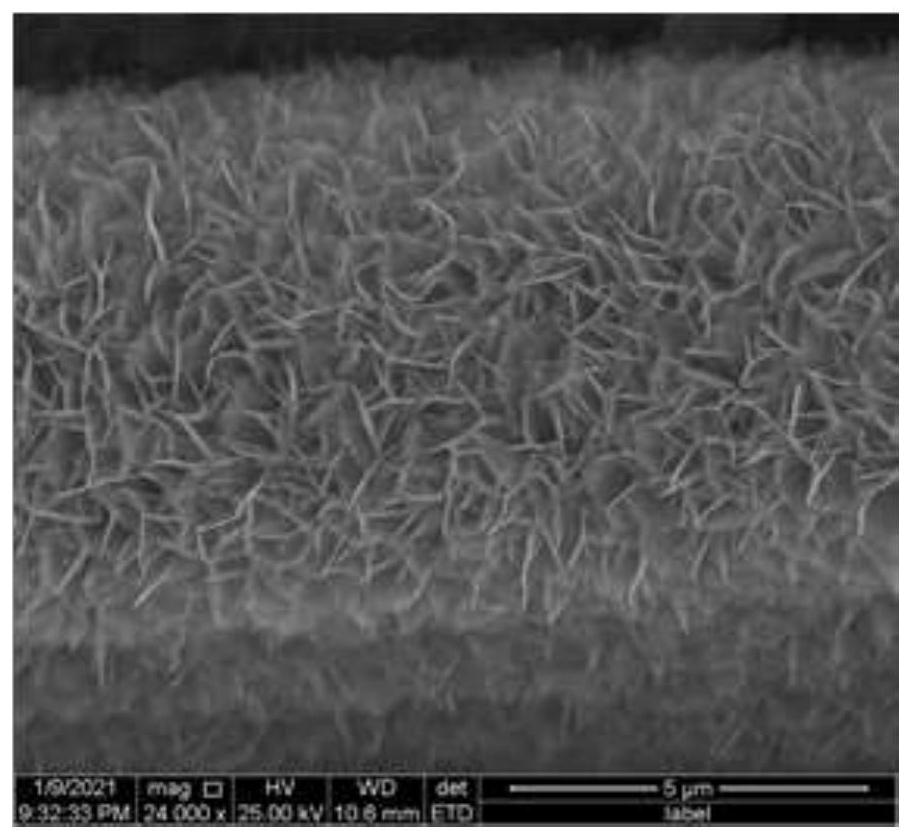
[0042] (1) Electrodeposition cobalt hydroxide nanosheet arrays on carbon cloth by electrodeposition method: add 0.01 mol of cobalt nitrate to 200 mL of deionized water and mix evenly to obtain a cobalt nitrate aqueous solution; use titanium mesh as the counter electrode and carbon cloth as the work Electrode, saturated calomel electrode is the reference electrode, each electrode is inserted into the cobalt nitrate aqueous solution, the electrodeposition potential is -0.1V, the electrodeposition time is 10min, and the cobalt hydroxide nanosheet array is obtained on the carbon cloth; deionized water The cobalt hydroxide nanosheet array was washed three times, then placed in a vacuum drying oven and dried at 70 °C for 12 h to obtain a carbon cloth grown with cobalt hydroxide nanosheet array, wherein the mass...
Embodiment 2
[0048] A preparation method of a cobalt phosphide electrode (N-CoP-CC) filled with nitrogen element containing phosphorus vacancies, the method steps are as follows:
[0049] The difference from Example 1 is that in step (4), 294 mg of V O -CoP-CC was placed in a tube furnace, under argon atmosphere, V was heated at a heating rate of 5 °C / min O -CoP-CC was heated to 300 ℃, and then passed ammonia gas for 1 hour, during which argon gas and ammonia gas were kept in the state of constant flow; among them, the flow rate of argon gas was 200sccm, and the flow rate of ammonia gas was 10sccm; At room temperature, a carbon cloth with cobalt phosphide nanosheet arrays grown with nitrogen filled with phosphorus vacancies, that is, a cobalt phosphide electrode with nitrogen filled with phosphorus vacancies (abbreviated as N-CoP-CC) is obtained. Other preparation methods are the same as in Example 1.
[0050] The N-CoP-CC was observed with an FEI Quanta 250 field emission scanning elect...
Embodiment 3
[0052] A preparation method of a cobalt phosphide electrode (F-CoP-CC) filled with phosphorus vacancies with fluorine element, the method steps are as follows:
[0053] The difference from Example 1 is that in step (4), a plasma treatment method was used to treat 294 mg of V O -Phosphorus vacancies in CoP-CC are filled with fluorine, and the specific conditions of plasma treatment are: pass CF into 4 , CF 4 The flow rate is 100sccm, the flow rate of argon gas is 80sccm, the effective power of the radio frequency power supply is 100W, and the processing time is 60min to obtain a carbon cloth with cobalt phosphide nanosheet arrays filled with fluorine element and phosphorus vacancies, that is, the carbon cloth with fluorine element filled with phosphorus vacancies. Cobalt phosphide electrode (abbreviated as: F-CoP-CC). Other preparation methods are the same as in Example 1.
[0054] The F-CoP-CC was observed with a FEI Quanta 250 field emission scanning electron microscope fr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| power | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com