Pectinase gene, pectinase, recombinant vector and application thereof in blueberry processing
A technology of recombinant vector and pectinase, applied in the direction of genetic engineering, application, plant gene improvement, etc., can solve the problems of limited products, unsuitable for large-scale application, etc., and achieve the effect of efficient degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
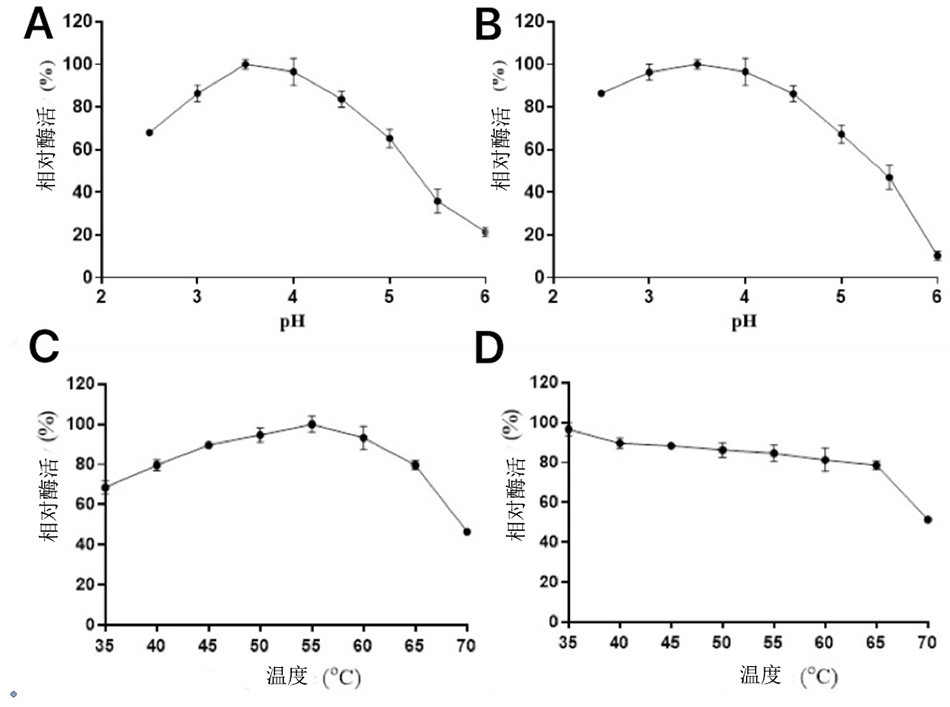
[0052] Example 1: Screening of pectinase-producing strains
[0053] The screening medium was prepared with pectin as the sole carbon source: pectin 5g / L, NaNO 3 2g / L, NaCl 1g / L, MgSO 4 1g / L, K 2 HPO 4 ·3H 2 O 1g / L, KH 2 PO 4 0.5g / L, bromophenol blue 0.01g / L. Take 10 g of fresh blueberry peel samples and shake them in 50 mL of sterile normal saline for 30 min. After standing, draw 4 mL of it and inoculate it into 100 mL of screening medium, and culture with shaking at 30 °C for 24 h. Take 0.5 mL of bacterial suspension for appropriate gradient dilution, and then take 0.1 mL of bacterial suspension of different dilutions and spread it on the primary screening medium plate (the composition of the primary screening medium plate is the addition of 2% agar to the screening medium) After culturing at 30°C for 48 hours, single colonies that can generate yellow circles on the bromophenol blue plate were selected for streak separation and purification. The purified strains wer...
Embodiment 2
[0056] Example 2: Strain identification and pectinase gene cloning
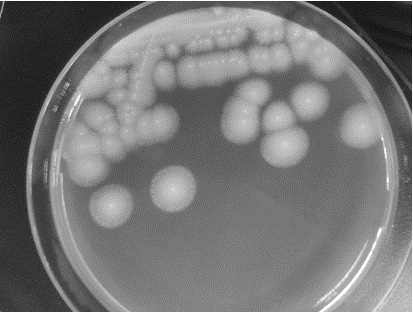
[0057] The strain LM1 was cultured on YPD solid medium at 28°C for two days, and the colony characteristics and cell shape were observed. The results are shown in image 3 , Aureobasidium pullulans LM1 screened by the present invention is preserved in the China Center for Type Culture Collection, and the preservation time is March 09, 2022. The address of the preservation unit is Wuhan, China, and the preservation number is CCTCC NO: M2022182.
[0058] The product was obtained by PCR amplification, and the sequence of 26S rDNA was obtained after the product was sequenced. The primers used for PCR amplification of D1 / D2 26SrDNA sequences are:
[0059] Upstream primer NL-1: 5'-GCATATCAATAAGCGGAGGAAAAG-3' (SEQ ID NO. 3);
[0060] Downstream primer NL-4: 5'-GGTCCGTGTTTCAAGACGG-3 (SEQ ID NO. 4).
[0061] The sequencing results were analyzed by the Basic Local Alignment Search Tool (BLAST) in the GenBank databas...
Embodiment 3
[0067] Example 3: Heterologous expression and purification of pectinase ZF05 in Pichia pastoris
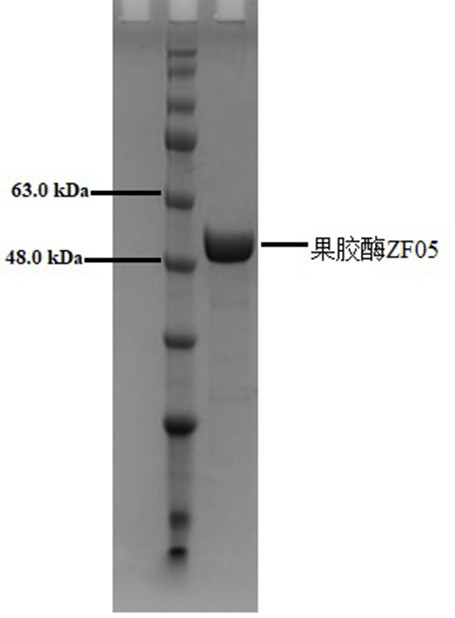
[0068] The pectinase gene ZF05 was linked with the pPIC9K Pichia vector to construct a recombinant vector, and electrotransformed according to the Pichia expression manual method. When a single colony was grown, it was screened and subjected to an induced expression experiment. The expression and purification of pectinase ZF05 were detected by polyacrylamide gel electrophoresis, and the results were as follows figure 1 As shown, the purified pectinase ZF05 showed a single band on the electrophoresis gel, and the position (about 49 kDa) matched the predicted molecular weight (49.30 kDa).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com