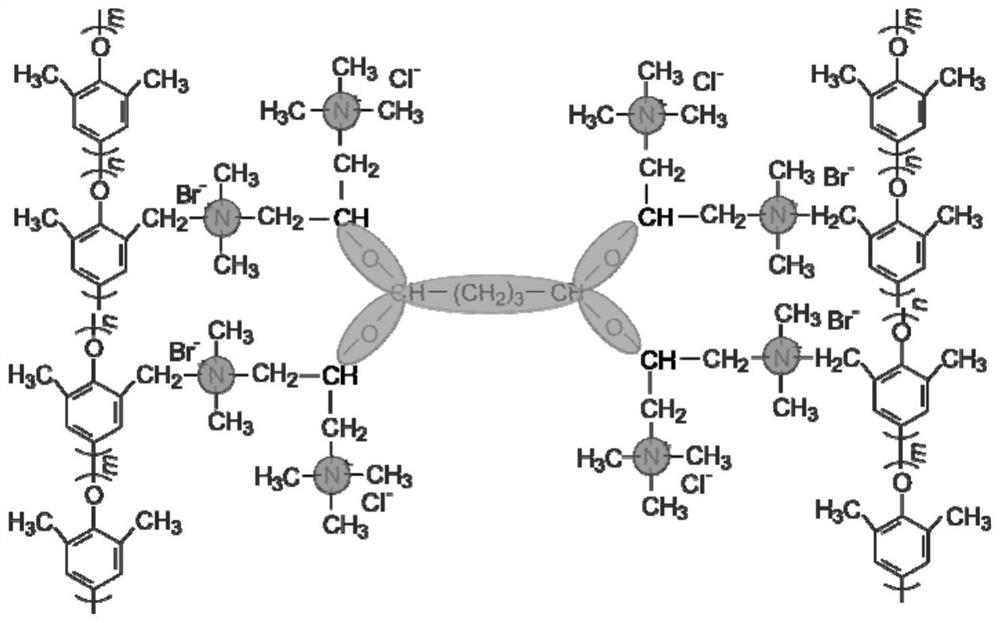
Star-shaped cross-linked alkaline polyelectrolyte and preparation method thereof
A polyelectrolyte and alkaline technology, which is applied in the field of star-shaped cross-linked alkaline polyelectrolyte and its preparation, can solve the problems of unsatisfactory single cell performance, limit the swelling of target materials, increase the ion conductivity, etc., and achieve good chemical Stability, promotion of ionic conductivity, and effect of increasing ion content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A star-shaped cross-linked alkaline polyelectrolyte for a fuel cell and a preparation method thereof, comprising the following steps:
[0048] (1) Preparation of cationic precursor: 4 g of 2,3-epoxypropyltrimethylammonium chloride was dissolved in 40 mL of ethanol at 60°C. After the dissolution was complete, excess dimethylamine aqueous solution was added to continue the reaction for 6 h. After the end, the solvent was removed with a rotary evaporator, and it was placed in a vacuum drying oven at 40° C. to continue drying for 48 hours to obtain a yellow oily final product, which was a cation precursor.
[0049] (2) Preparation of brominated polyphenylene ether: Weigh 4g of polyphenylene ether in 50mL of chlorobenzene, stir and dissolve at 120°C, add 10g of brominating agent N-bromosuccinimide, dissolve, and heat up to At 135°C, 0.6 g of azobisisobutyronitrile was slowly added, and after reacting at 135°C for 4 hours, the product was precipitated in ice methanol, washed ...
Embodiment 2
[0053] A star-shaped cross-linked alkaline polyelectrolyte for fuel cells and a preparation method thereof:
[0054] It is basically the same as Example 1, except that the content of the cationic precursor in step (3) is 0.45 mmol, and the content of glutaraldehyde is 0.11 mmol.
[0055] The ion exchange capacity of the star-shaped cross-linked alkaline polyelectrolyte was measured to be 3.15 mmol g -1 , the gel degree was 92.1%, the room temperature swelling rate was 8.20%, the room temperature ionic conductivity was 32.5 mS / cm, and the mass loss rate and ionic conductivity loss rate after 30 days of alkali resistance stability were 17.3% and 28.6%, respectively.
Embodiment 3
[0078] A star-shaped cross-linked alkaline polyelectrolyte for fuel cells and a preparation method thereof:
[0079] It is basically the same as Example 1, the difference is: the content of the cation precursor in step (3) is 0.60 mmol, and the content of glutaraldehyde is 0.15 mmol. The ion exchange capacity of the membrane was measured to be 3.59 mmol g -1 , the gel degree was 92.0%, the room temperature swelling rate was 14.8%, the room temperature ionic conductivity was 51.6 mS / cm, and the mass loss rate and ionic conductivity loss rate after 30 days of alkali resistance stability were 14.8% and 24.2%, respectively.
[0080] The above results show that the preparation process of the star-shaped cross-linked alkaline polyelectrolyte for fuel cells disclosed in the embodiment of the present invention does not involve the use of the highly toxic substance chloromethyl ether, and can achieve the purpose of adjustable ion exchange capacity. as attached figure 1 As shown, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| gel fraction | aaaaa | aaaaa |
| gel fraction | aaaaa | aaaaa |
| gel fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com