Culture medium for culturing living biological medicine and application of culture medium
A technology of living organisms and culture medium, applied in the field of microorganisms, can solve the problems of limited proliferation promotion of probiotics, achieve significant drug effects, prevent or treat non-alcoholic fatty liver disease, and promote growth and proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 - Preparation of culture medium
[0047] Formula 1: By mass percentage: peptone 1%, beef extract 1%, lactulose 0.6%, oligomannose 1.6%, raffinose 0.8%, yeast extract 0.5%, diammonium hydrogen citrate 0.2% %, K 2 HPO 4 ·3H 2 O 0.2%, MgSO 4 ·7H 2 O 0.058%, MnSO 4 0.019%, Tween-80 0.1%, L-cysteine 0.1%, riboflavin 0.1%, asparagine 0.1%, and the balance is water.
[0048] Formula 2: By mass percentage: peptone 0.6%, beef extract 1.5%, lactulose 0.4%, mannose oligosaccharide 2%, raffinose 0.6%, yeast extract 0.8%, diammonium hydrogen citrate 0.05% %, K 2 HPO 4 ·3H 2 O 0.5%, MgSO 4 ·7H 2 O 0.1%, MnSO 4 0.01%, Tween-80 0.3%, L-cysteine 0.03%, riboflavin 0.05%, asparagine 0.15%, and the balance is water.
[0049] Formula 3: By mass percentage: peptone 1.5%, beef extract 0.5%, lactulose 0.8%, oligomannose 1.2%, raffinose 1%, yeast extract 1%, diammonium hydrogen citrate 0.3% %, K 2 HPO 4 ·3H 2 O 0.05%, MgSO 4 ·7H 2 O 0.02%, MnSO 4 0.08%, Twee...
Embodiment 2
[0065] The present embodiment provides a culture of living biological medicine LBPs1 (combination of Lactobacillus reuteri LR08 and Lactobacillus paracasei LC86, weight ratio 3:1) for preventing or treating asthma, and the preparation method is as follows:
[0066] A. Activation: Take out Lactobacillus reuteri LR08 and Lactobacillus paracasei LC86 stored in a 40% glycerol tube in a -80°C refrigerator. After the glycerol tube is thawed, mix the samples at a weight ratio of 3:1. Pipet 2% of the sample in a sterile operating table and add it to 20 mL of modified MRS medium. After culturing at 37 °C for 18 h, take out and centrifuge (8000 g for 10 min) to obtain the separated bacterial slurry, which is then used in 9 mL. Resuspend in sterile physiological saline and shake evenly to obtain bacterial liquid.
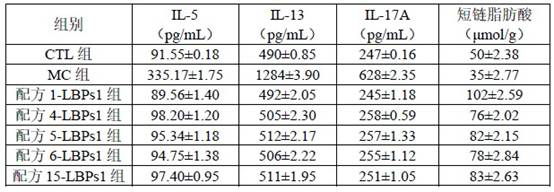
[0067] B. Cultivation: The culture medium of formula 1 to formula 15 in Example 1 was used to cultivate the living biopharmaceutical LBPs1. Dispense the medium into centrifug...
Embodiment 3
[0092] This example provides a culture of living biological drug LBPs2 (combination of Lactobacillus acidophilus LA88 and Lactobacillus rhamnosus LRa05, weight ratio 1:1) for the prevention or treatment of non-alcoholic fatty liver disease.
[0093] A. Activation: Refer to Step A of Example 2.
[0094] B. Cultivation: Refer to step B of Example 2.
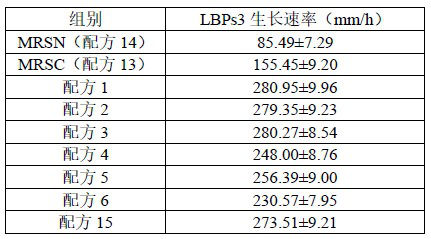
[0095] C Colony growth rate comparison: with reference to step C of Example 2, the results are shown in Table 4.
[0096] Table 4
[0097]
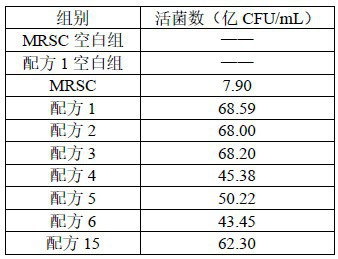
[0098] D Bacterial proliferation ability comparison: with reference to step D of Example 2, the results are shown in Table 5.
[0099] table 5
[0100]
[0101] It can be seen from the results in Table 4 and Table 5: on the premise that the total amount is consistent, the present invention combines lactulose, oligomannose and raffinose, which has a more significant effect than the formula lacking one component. Compared with the conventional medium (MRSC), the growth rate of the colo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com