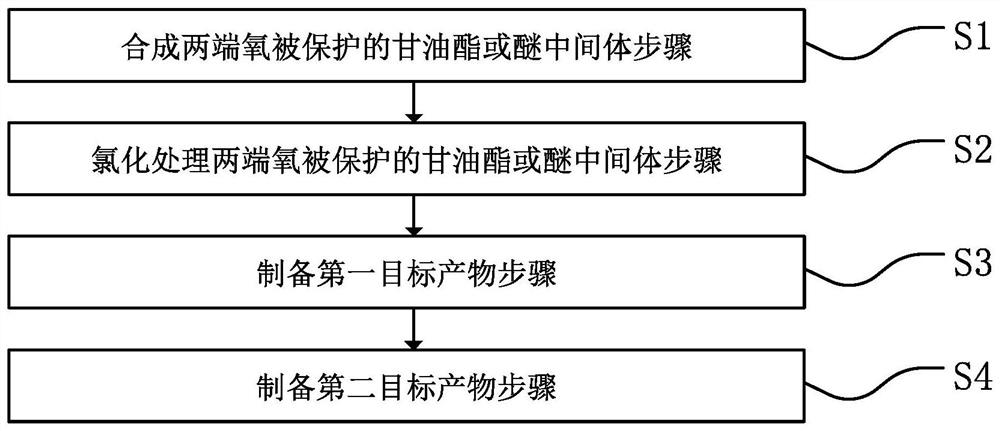
Preparation method of 2-MCPD, D5-2-MCPD and 13C3-2-MCPD
A target and intermediate technology, applied in the field of high-purity preparation, can solve the problems of complex production process, complex product, high production cost, etc., and achieve the effect of low process cost and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Synthesis of 2-chloro-1,3-propanediol (2-MCPD)
[0048]
[0049] Step 1: Add glycerol (15g, 0.16mol, 1.0eq), dichloromethane (300mL), triethylamine (48.5g, 0.48mol, 3.0eq) into the reaction kettle, cool down to -10°C, and dropwise add benzyl Acid chloride (46.1 g, 0.33 mol, 2.05 eq), maintaining the reaction temperature at -10-0 °C. After the dropwise addition was completed, the reaction was continued for 60 minutes. Water (150 mL) was added, and the mixture was fully stirred for 10 minutes, the upper aqueous phase was separated, and the lower organic phase was concentrated to dryness.
[0050] Step 2: Add the concentrated solution of Step 1 and dichloromethane (300mL) into the reactor, slowly add phosphorus pentachloride (50g, 0.24mol, 1.5eq), maintain the reaction temperature at 15-20°C, and stir the reaction for 5 hours . Slowly add water (150 mL), stir well for 10 minutes, separate the upper aqueous phase, and concentrate the lower organic phase to d...
Embodiment 2
[0052] Example 2 Synthesis of 2-chloro-1,3-propanediol stearic acid diester (2-MCPD stearic acid diester)
[0053]
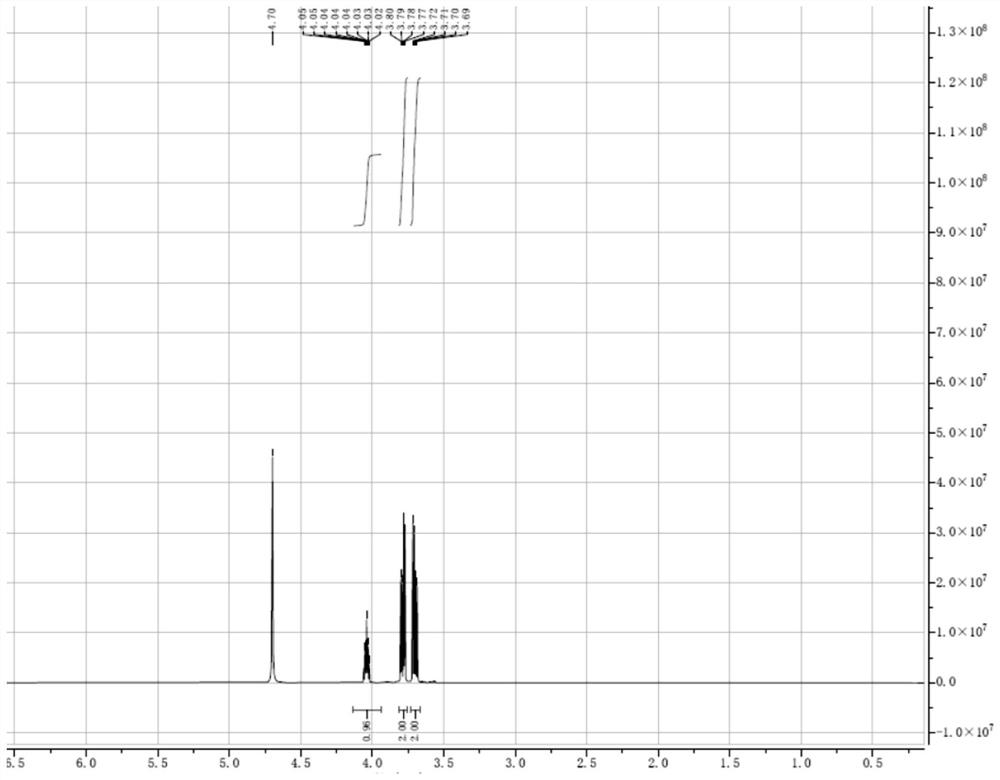
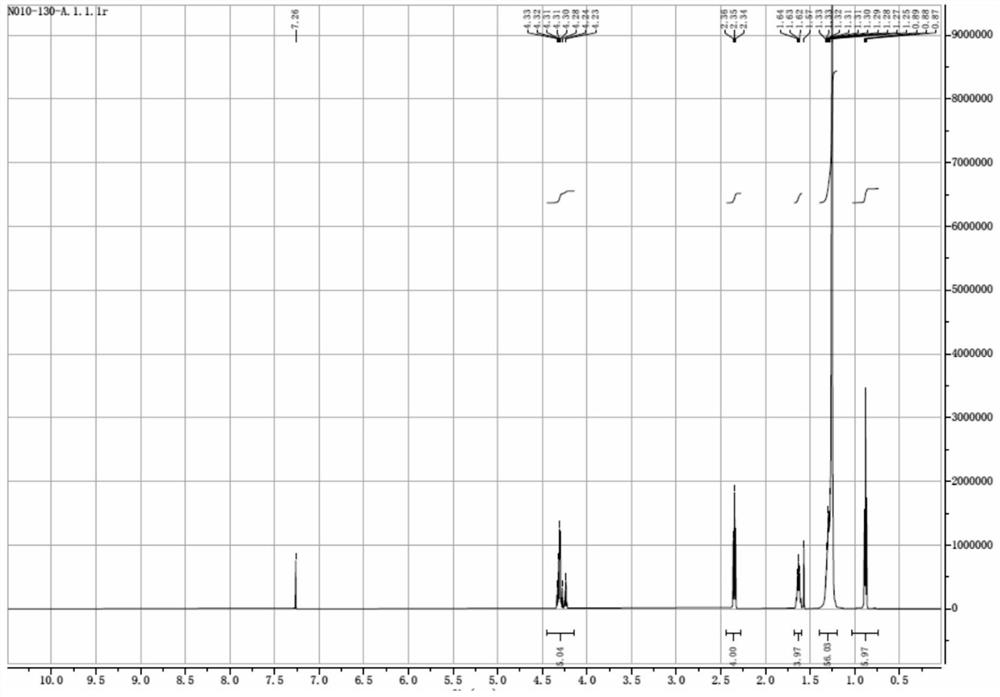
[0054] 2-Chloro-1,3-propanediol (2-MCPD) (5g, 0.045mol, 1.0eq), stearic acid (25.6g, 0.090mol, 2.0eq), 4-dimethylformaldehyde in Step 3 of Example 1 were mixed Aminopyridine (DMAP) (0.55g, 0.0045mol, 0.1 eq), dicyclohexylcarbodiimide (DCC) (22.2g, 0.11mol, 2.4eq), and dichloromethane (150mL) were added to the reaction kettle, at room temperature Stir for 5-6 hours. Water (150 mL) was added, fully stirred for 10 minutes, the upper aqueous phase was separated, the lower organic phase was concentrated to dryness, methanol (100 mL) was added, stirred for 1 hour, and filtered to obtain 2-chloro-1,3-propanediol stearic acid diester ( 2-MCPD stearic acid diester) pure product 26.2g (see NMR spectrum image 3 ), the yield was 90.2%.
Embodiment 3
[0055] Example 3 Synthesis of 2-chloro-1,3-propanediol (2-MCPD)
[0056]
[0057] Step 1: Add glycerol (3g, 0.033mol, 1.0eq), dichloromethane (60mL), triethylamine (9.9g, 0.098mol, 3.0eq) into the reaction kettle, cool down to -10°C, slowly add tert-butyl Diphenylchlorosilane (18.6g, 0.068mol, 2.05eq), maintaining the reaction temperature at -10-0°C. After the addition was completed, the reaction was continued for 60 minutes. Water (50 mL) was added, and the mixture was fully stirred for 10 minutes, the upper aqueous phase was separated, and the lower organic phase was concentrated to dryness.
[0058] Step 2: Add the concentrated solution of step 1 and dichloromethane (60mL) into the reaction kettle, slowly add phosphorus trichloride (6.8g, 0.050mol, 1.5eq), maintain the reaction temperature at 15-20°C, and stir for 5 Hour. Slowly add water (60 mL), stir well for 10 minutes, separate the upper aqueous phase, and concentrate the lower organic phase to dryness.
[0059] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com