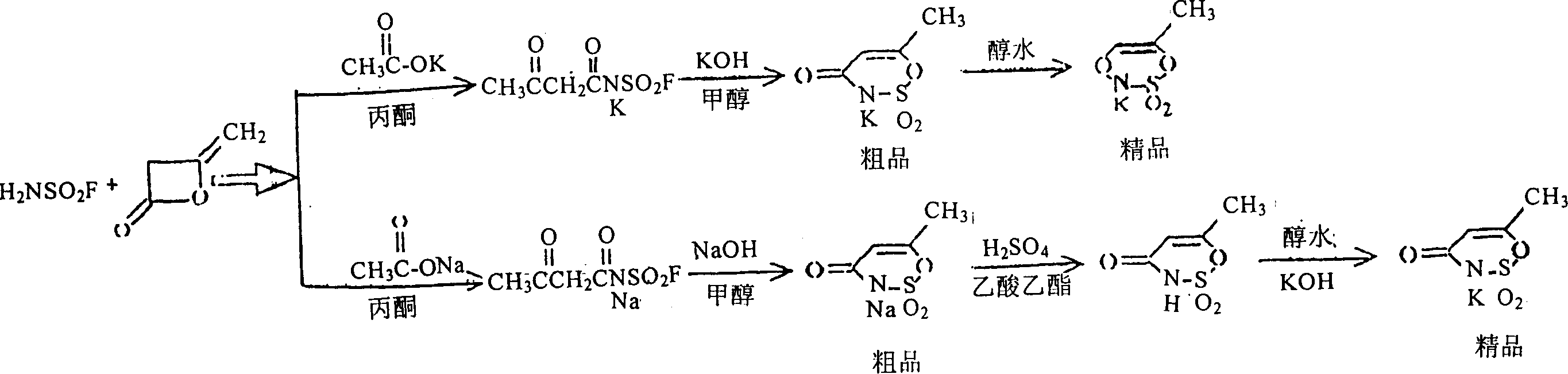
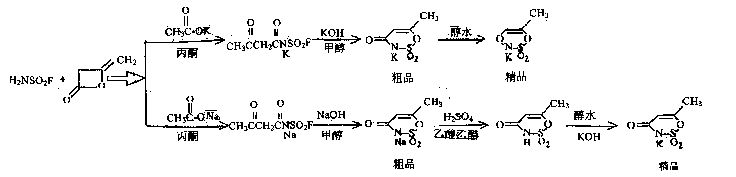
Method for preparing 6-methyl-1,2,3-oxazine-4(3H)-ketone-2,2-dioxopotassium salt
A technology of potassium dioxooxide and oxthiazine is applied in the field of preparing 6-methyl-1, can solve the problems of large product loss, high reaction temperature, short reaction steps and the like, and achieves low cost, simple synthesis and purification yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] 1. Preparation of acetoacetamidosulfonyl fluoride potassium salt:
[0064] Add 98g (1mol) of potassium acetate to 500ml of acetone, add 99g (1mol) of sulfamoyl fluoride at about 0°C under stirring, then add 84g (1mol) of diketene dropwise within 30 minutes under the same conditions, and continue to Stir at 0°C for 30 minutes, then rise to room temperature and stir at 20-25°C for 4 hours, filter, and wash twice with 40ml of acetone to obtain 200g of white crystalline solid, y=90%. It can be directly used in the next reaction.
[0065] 2. Preparation of crude product of 6-methyl-1,2,3-oxathiazin-4(3H)-one-2,2-dioxyl potassium salt
[0066] Add the above acetoacetamidosulfonyl fluoride potassium salt to a solution of 60g KOH in 300ml methanol, stir at room temperature at 20-25°C for 4 hours, filter, and wash with 20ml methanol to obtain 194g of crude product, y=97%.
[0067] 3. Preparation of 6-methyl-1,2,3-oxathiazin-4(3H)-one-2,2-dioxopotassium salt
Embodiment 2
[0070] 1. Preparation of acetoacetamidosulfonyl fluoride sodium salt:
[0071] Add 70g (1mol) of sodium acetate to 500ml of acetone, add 99g (1mol) of sulfamoyl fluoride at about 0°C under stirring, then add 84g (1mol) of diketene dropwise within 30 minutes under the same conditions, and continue to Stir at 0°C for 30 minutes, then rise to room temperature and stir at 20-25°C for 4 hours, filter, and wash twice with 40ml of acetone to obtain 200g of white crystalline solid, y=90%. It can be directly used in the next reaction.
[0072] 2. Preparation of crude product of 6-methyl-1,2,3-oxathiazin-4(3H)-one-2,2-dioxosodium salt
[0073] Add the above sodium acetoacetamidosulfonyl fluoride to a solution of 60g KOH in 300ml methanol, stir at room temperature at 20-25°C for 4 hours, filter, and wash with 20ml methanol to obtain 194g of crude product, y=97%.
[0074] 3. Preparation of 6-methyl-1,2,3-oxathiazin-4(3H)-one-2,2-dioxopotassium salt
[0075] Dissolve 100g of crude acesu...
Embodiment 3
[0077] Prepare 6-methyl-1,2,3-oxathiazin-4(3H)-ketone-2,2-dioxo potassium salt by the method of Example 1, just replace the potassium succinate in Example 1 potassium acetate.
[0078] 1. Preparation of acetoacetamidosulfonyl fluoride potassium salt:
[0079] Add 194g (1 equivalent) of potassium succinate to 500ml of acetone, add 99g (1mol) of sulfamoyl fluoride at about 0°C under stirring, then add 84g (1mol) of diketene dropwise within 30 minutes under the same conditions, and add Continue to stir at 0°C for 30 minutes, then rise to room temperature and stir at 20-25°C for 4 hours, filter, and wash twice with 40ml of acetone to obtain 190g of white crystalline solid, y=85%. It can be directly used in the next reaction.
[0080] 2. Preparation of crude product of 6-methyl-1,2,3-oxathiazin-4(3H)-one-2,2-dioxyl potassium salt
[0081] The above-mentioned acetoacetamidosulfonyl fluoride potassium salt was added to a solution of 60g KOH in 300ml methanol, stirred at room tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com