High temperature lipase, coding gene order and uses thereof
A lipase, high temperature technology, applied in genetic engineering, plant genetic improvement, applications, etc., can solve problems such as poor thermal stability, and achieve the effect of high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
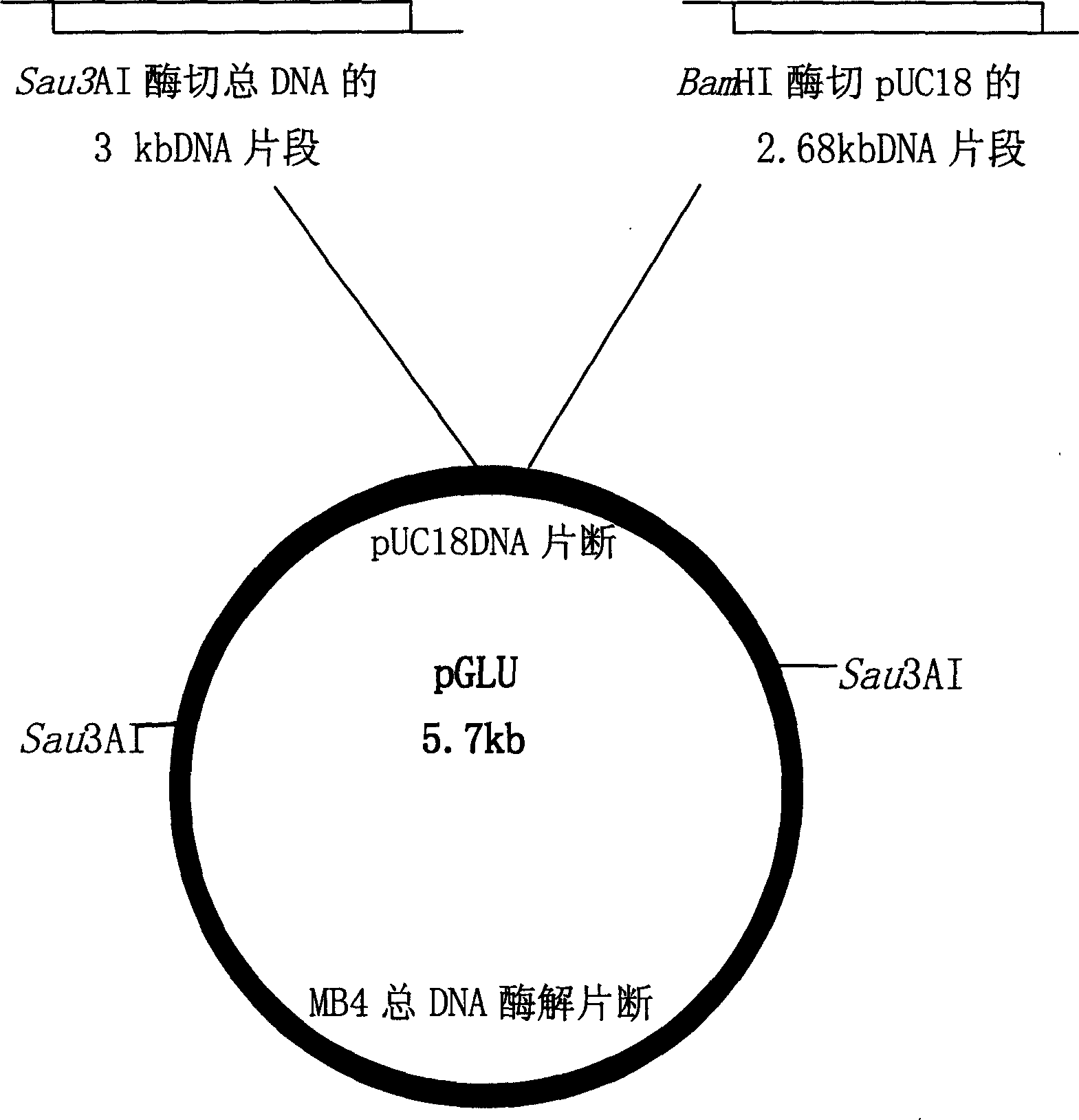
[0021] Extraction of total DNA from thermophilic anaerobic bacteria MB4
[0022]The thermophilic anaerobic bacterium Thermoanaerobactertengcongensis MB4 isolated from Tengchong Hot Spring, Yunnan, China, was used to get 20 grams of fresh wet thallus, suspend in 10 milliliters of 50mM Tris buffer (pH8.0), add a small amount of lysozyme and 8 milliliters of 0.25mM EDTA ( pH8.0), mix well and place at 37°C for 20min, then add 2 ml of 10% SDS, place at 55°C for 5min, extract with equal volume of phenol and chloroform respectively, take the last supernatant solution, add 2 times Volume ethanol, recover DNA, wash with 70% and absolute ethanol respectively, dissolve the precipitate in 0.5 ml TE buffer solution (pH8.0, 10 mM Tris, 1 mM EDTA), add 10 mg / ml RNase 3 μl, incubate at 37 ° C for 1 hour, use etc. The volume of phenol and chloroform were extracted once, and the supernatant was added with 2 times the volume of ethanol to recover the DNA, washed with 70% and absolute ethanol re...
Embodiment 2
[0028] Purification and Characterization of Recombinant Lipase
[0029] The cells of the recombinant bacteria E.coli DH5αLIP were suspended in 50mM phosphate buffer (pH7), the cells were disrupted by ultrasonic waves, and the centrifuged supernatant was the crude enzyme solution of the recombinant lipase. The supernatant enzyme solution was heated at 70oC for 15 minutes, centrifuged to remove denatured protein, the supernatant enzyme solution was purified by ion exchange column chromatography, hydroxyapatite adsorption column chromatography and PAGE preparative electrophoresis, and the obtained enzyme preparation was prepared in SDS- A band appears on the PAGE. The basic properties of this recombinant lipase were determined using known standard methods of protein chemistry. The molecular weight of the recombinant enzyme measured by SDS-PAGE is 42,000 Daltons, which is similar to the theoretically calculated molecular weight (45,200 Daltons); the reaction temperature of the re...
Embodiment 3
[0031] Hydrolyzed olive oil with recombinant lipase
[0032] Add an appropriate amount of enzyme solution to 50% olive oil solution (prepared with pH7, 0.2M sodium phosphate buffer), heat up to 60° C., and stir at 200 rpm for 16 hours. The oil phase contained fatty acid and the water phase contained glycerol as determined by chromatography, which indicated that the recombinant lipase could hydrolyze olive oil to produce glycerol and fatty acid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com