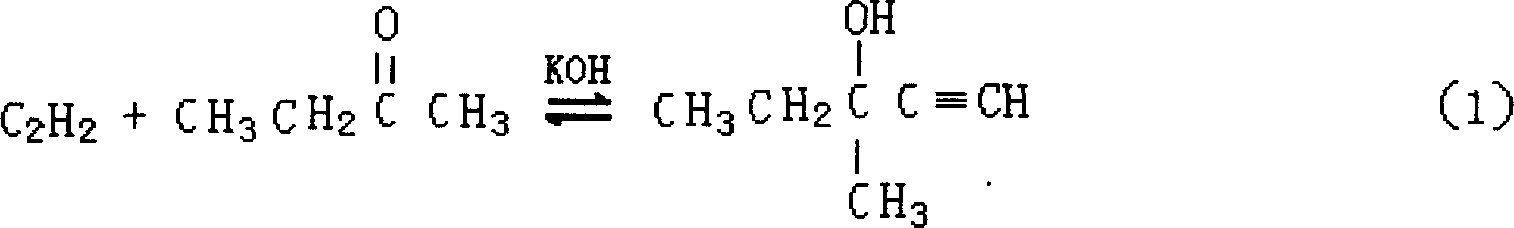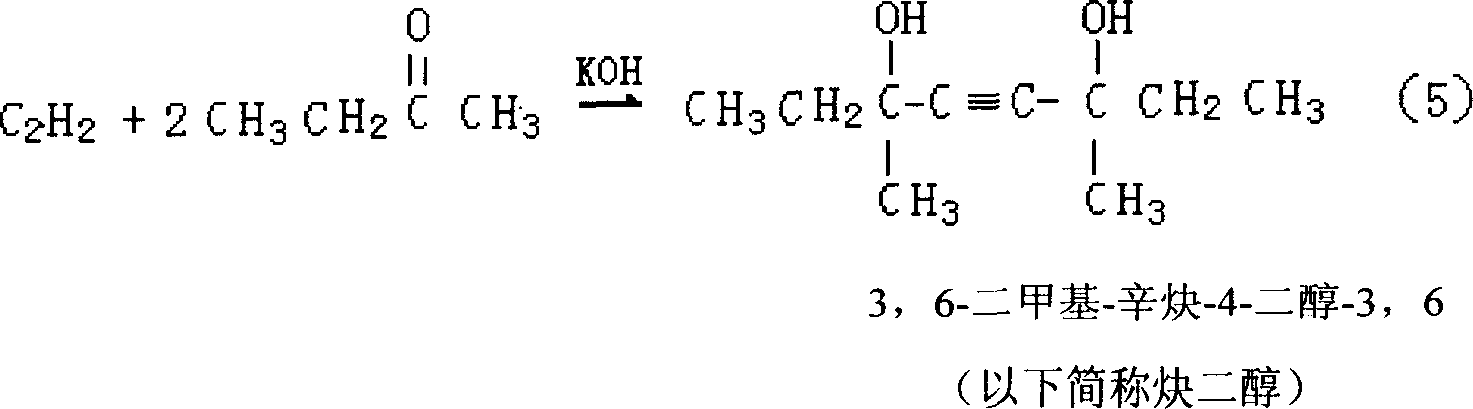Method for synthesizing alkynol by ketone and acetylene
A technology of acetylene and acetylenic alcohol, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of hydroxyl compounds, etc. It can solve the problems of limited application range, low reaction conversion rate, and low space velocity, etc., and it is easier to control the reaction temperature , the reaction temperature is stable, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6
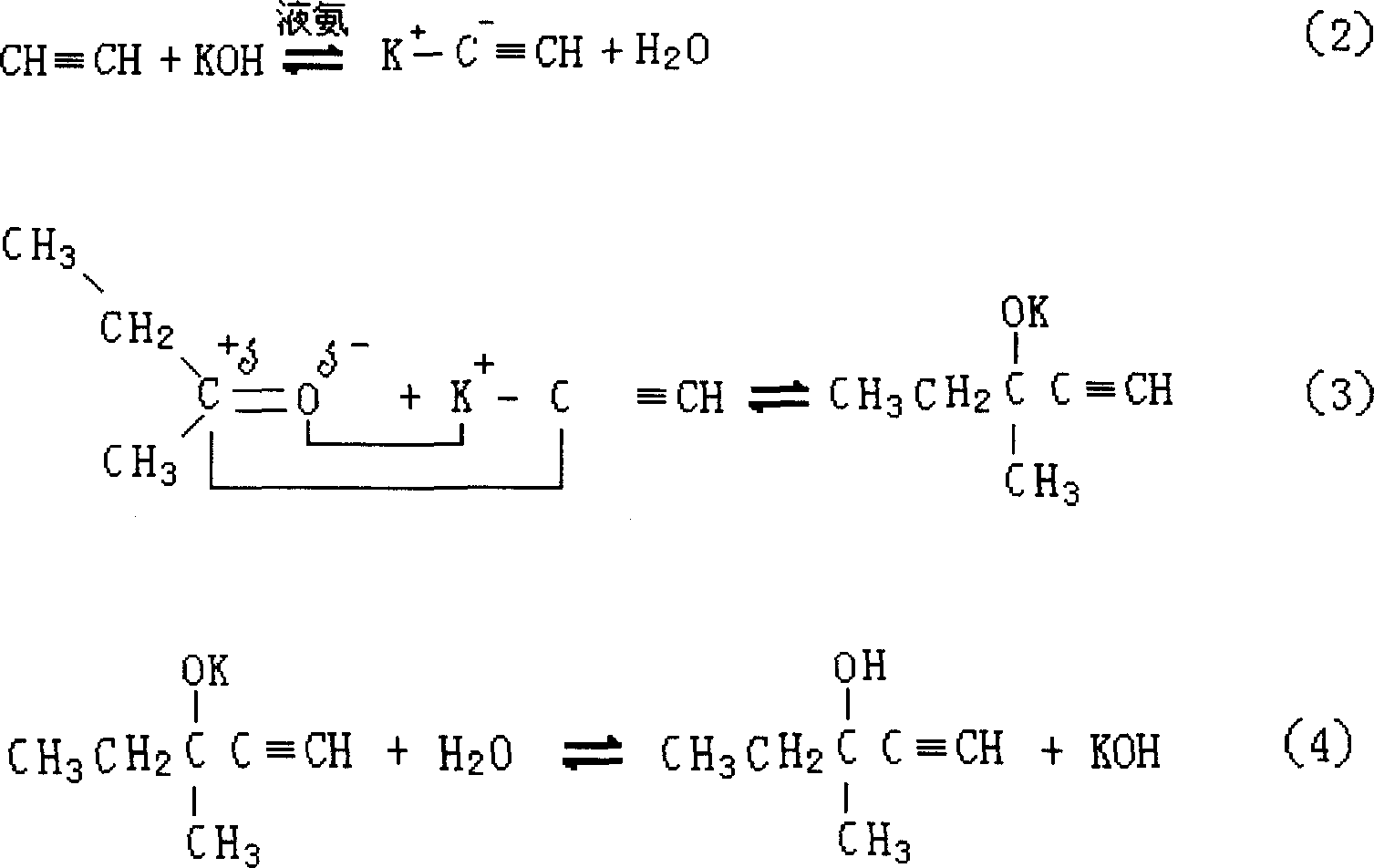
[0039] Using methyl ethyl ketone and acetylene as raw materials, potassium hydroxide as catalyst (potassium hydroxide is added in the form of aqueous solution), and liquid ammonia as acetylene solvent, the synthesis of methylpentynol is carried out in an autoclave under certain pressure and temperature conditions. Specifically: ① use nitrogen with a purity of 99v% to replace the reaction system three times under a pressure of 0.3-0.5 MPa; ② feed liquid ammonia into the autoclave; ③ feed acetylene into the autoclave once to dissolve it in the liquid ammonia . When the reaction was in progress, the methyl ethyl ketone and potassium hydroxide aqueous solution were fed continuously dropwise. The temperature required for the reaction is controlled by the circulating liquid in the wall jacket of the reactor, and the reaction liquid is continuously stirred during the reaction. The specific reaction conditions and results are shown in Table 1. As can be seen from the data in Table 1...
Embodiment 7~12
[0043] Using acetone and acetylene as raw materials, potassium hydroxide alkoxide aqueous solution as catalyst, and liquid ammonia as acetylene solvent, the synthesis of methyl butynol is carried out in an autoclave under certain pressure and temperature conditions. The implementation of the reaction process is the same as in Example 1. The specific reaction conditions and results are shown in Table 2. As can be seen from the data in Table 2, the reaction results of Examples 8-10 are better, indicating that the reaction conditions of Examples 8-10 are more suitable for the synthesis reaction of methyl butynol.
Embodiment 13~16
[0047] Using ketone and acetylene as raw materials and potassium hydroxide as a catalyst in a 10L reactor, the specific reaction conditions and results are shown in Table 3.
[0048] Example
[0049] Note: The reactor is a 2.0 liter autoclave with agitator
[0050] Example
[0051] Note: The reactor is a 2.0 liter autoclave with agitator
[0052] Example
[0053] Among them: MH is 6-methyl-hept-5-en-2-one, DMGA is 6,10-dimethyl-undec-5,9-dien-2-one, TMFA is 6,10, 14-Trimethyl-5,9,13-pentadeca-trien-2-one, TMPT is 6,10,14-trimethyl-pentadeca-2-one.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com