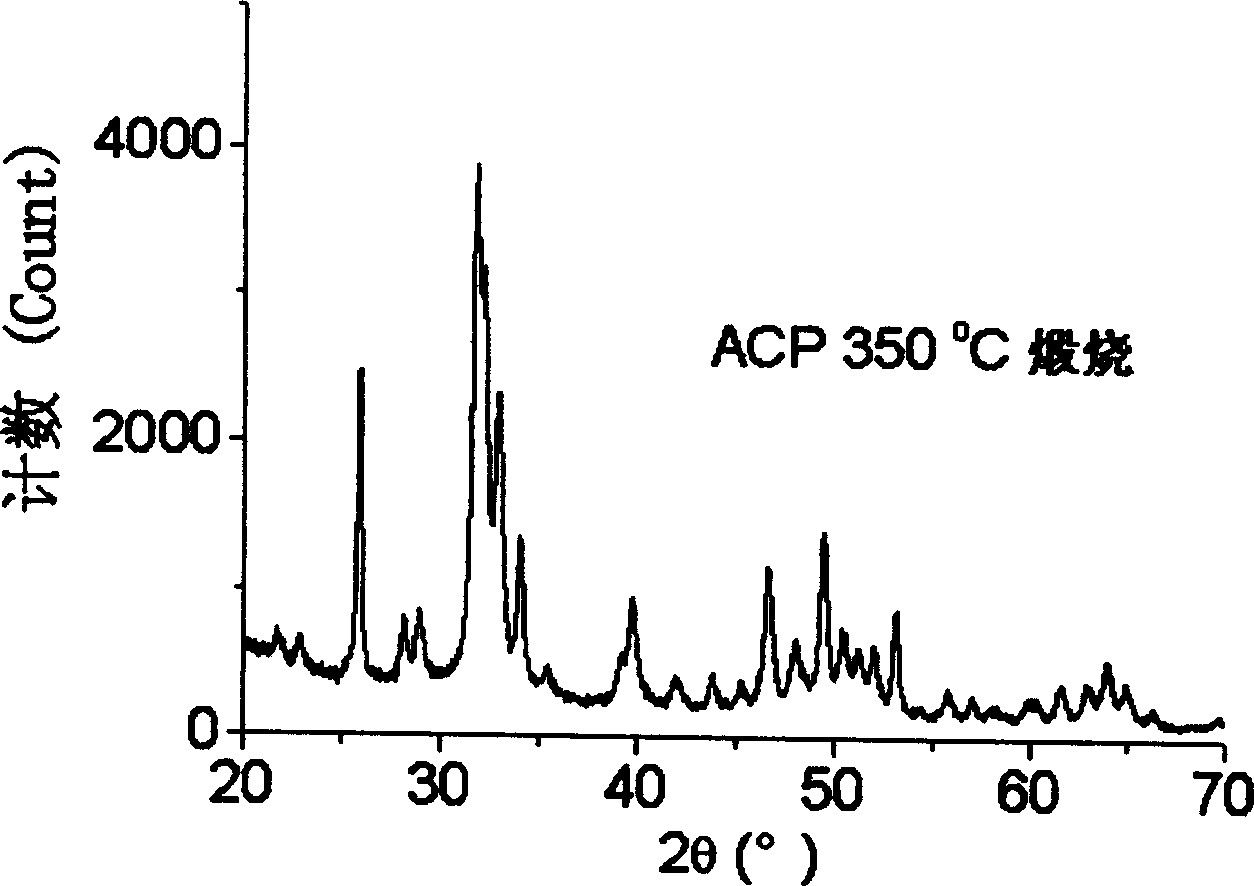
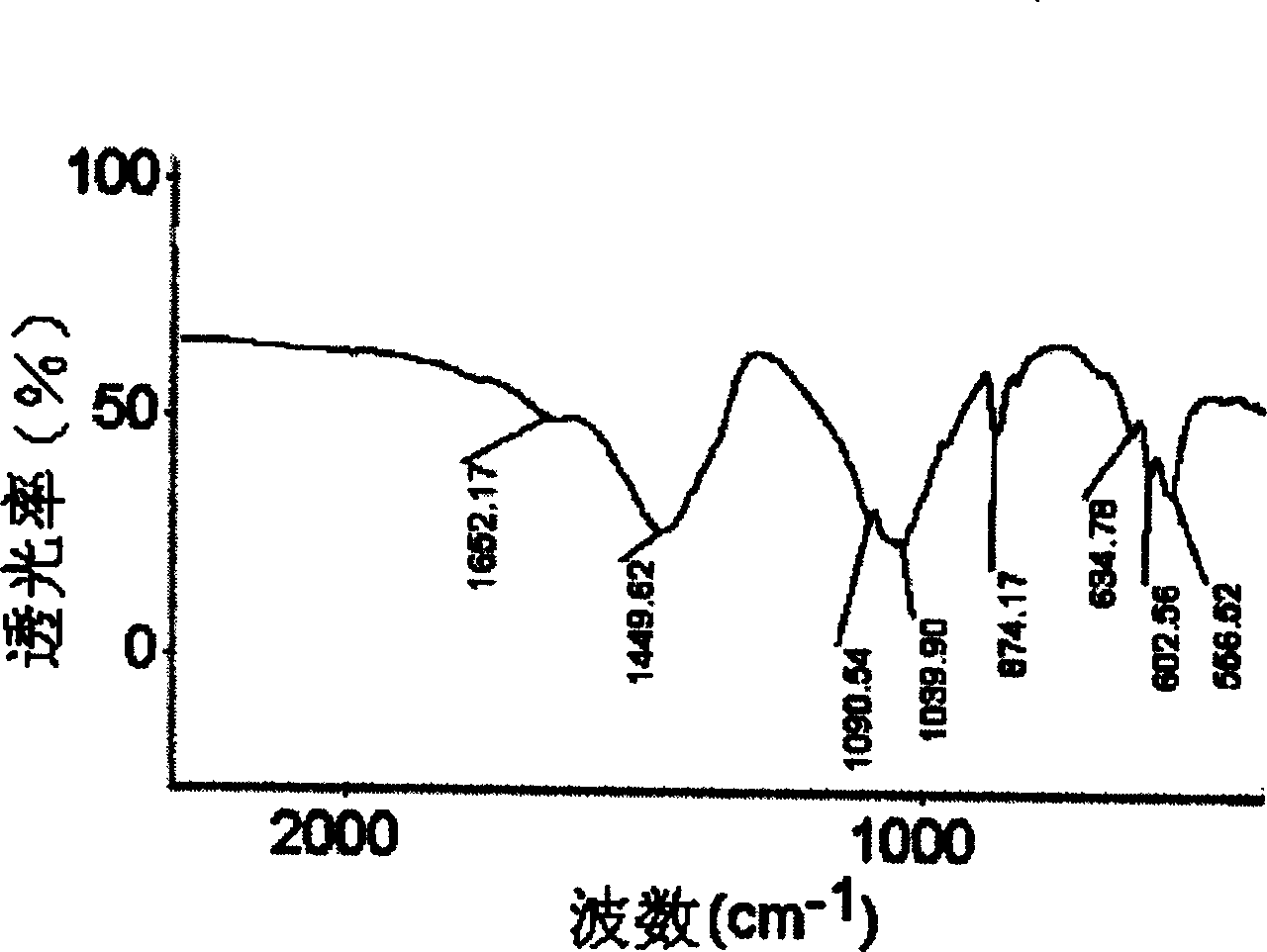
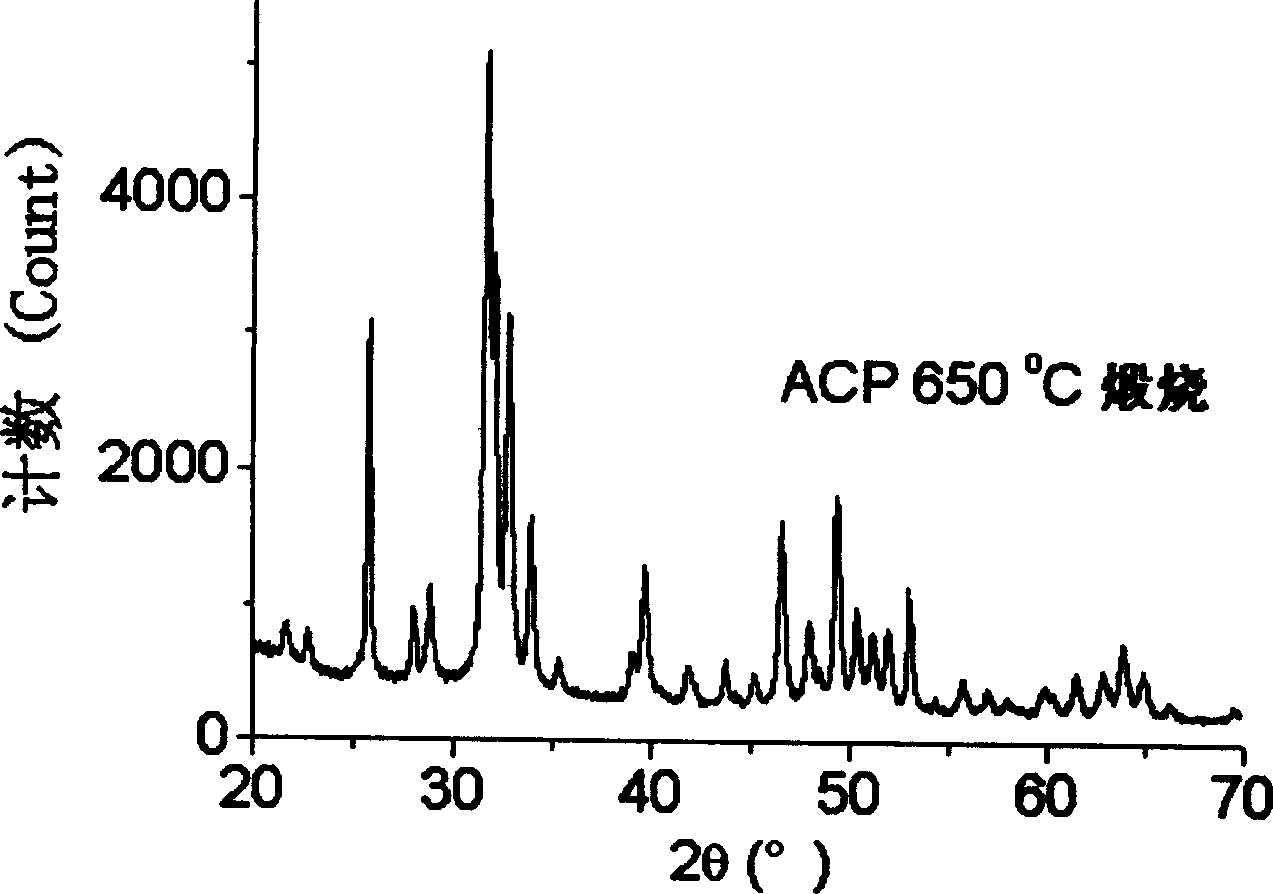
Carbonic acid type high activity partially crystallized calcium phosphate and its prepn
A crystalline calcium phosphate, high-activity technology, applied in chemical instruments and methods, phosphorus compounds, medical science, etc., can solve the problems affecting the speed and degree of hydration reaction, affecting the performance of powder, and the powder is prone to agglomeration, etc. Achieve the effect of improving reactivity and solubility, excellent reactivity and biodegradability, and good degradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Calcium acetate (CH 3 COO) 2 Ca·H 2 O is dissolved in water, prepared into a solution with a concentration of 2.3M, and according to the molar ratio of surfactant and calcium of 0.01, sodium lauryl sulfate is added to form A solution; diammonium hydrogen phosphate (NH 4 ) 2 HPO 4 Dissolve in water and make solution B with a concentration of 0.5M; according to the molar ratio of magnesium to calcium of 0.014 and the molar ratio of strontium to calcium of 0.028, add magnesium chloride MgCl to solution A in turn 2 ·6H 2 O, strontium nitrate Sr(NO 3 ) 2 , form C solution; add sodium carbonate Na 2 CO 3 , forming D solution; according to the calcium-phosphorus molar ratio being 1.63, the C solution is quickly introduced into the constantly stirring D solution with a catheter, and potassium hydroxide KOH solution is added to adjust and maintain the pH value of the reaction solution at 12, and the reaction time is 10min. Then centrifuge the reaction product, filter it...
Embodiment 2
[0037] CaCl 2 ·6H 2 O is dissolved in water, prepared into a solution with a concentration of 0.5M, and according to the molar ratio of surfactant and calcium of 0.01, polyethylene glycol (molecular weight 6000) is added to form A solution; dipotassium hydrogen phosphate K 2 HPO 4 Soluble in water and make solution B with a concentration of 1.2M; according to the molar ratio of zinc and calcium being 0.04, the molar ratio of magnesium and calcium being 0.024, and the molar ratio of strontium and calcium being 0.046, add zinc acetate to solution A in sequence (CH 3 COO) 2 Zn, magnesium chloride MgCl 2 ·6H 2 O, strontium nitrate Sr(NO 3 ) 2 , form C solution; according to the molar ratio of carbonate and phosphorus is 0.5, add potassium carbonate K in B solution 2 CO 3 , to form D solution; according to the calcium-phosphorus molar ratio of 1.3, the C solution was slowly added dropwise to the constantly stirring D solution, and at the same time, ammonia water was added ...
Embodiment 3
[0039] Calcium acetate (CH 3 COO) 2 Ca·H 2 O was dissolved in water and prepared into a solution with a concentration of 2M. According to the molar ratio of surfactant and calcium of 0.035, hexadecyltrimethylammonium bromide (C 19 h 42 NBr), form A solution; Triammonium phosphate (NH 4 ) 3 PO 4 ·3H 2 O is dissolved in water and prepared as B solution with a concentration of 0.5M; magnesium acetate (CH 3 COO) 2 Mg·4H 2 O, form C solution; Add ammonium carbonate (NH 4 ) 2 CO 3 ·H 2 O, form D solution; According to the calcium-phosphorus molar ratio is 1.6, the C solution is quickly introduced into the constantly stirring D solution with a catheter, and sodium hydroxide solution is added to adjust and maintain the pH value of the reaction solution at 13, and the reaction time is 12h. Then the reaction product was centrifuged, pumped, washed twice with deionized water, and then washed once with absolute ethanol. Ultrasonic treatment was introduced during the washing p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com