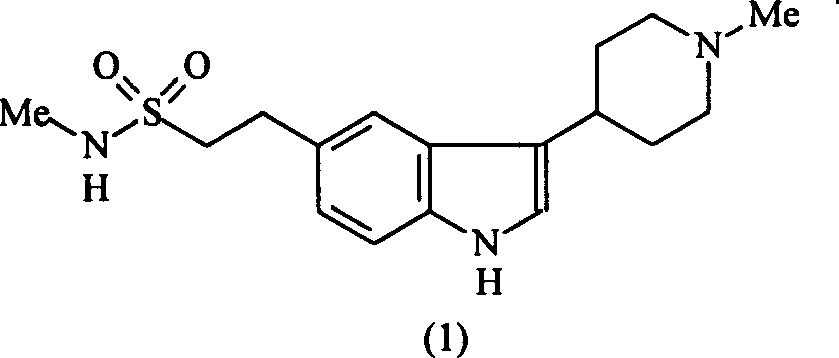
Improvement of preparation of Naratriptan
A synthetic method, methyl technology, applied in the field of improved method of Naratriptan preparation, can solve problems such as high operating cost, preparation without commercial supply, difficulties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
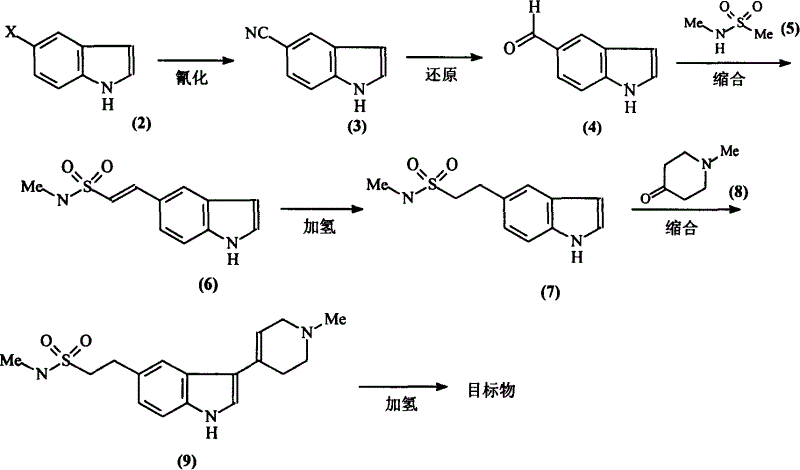
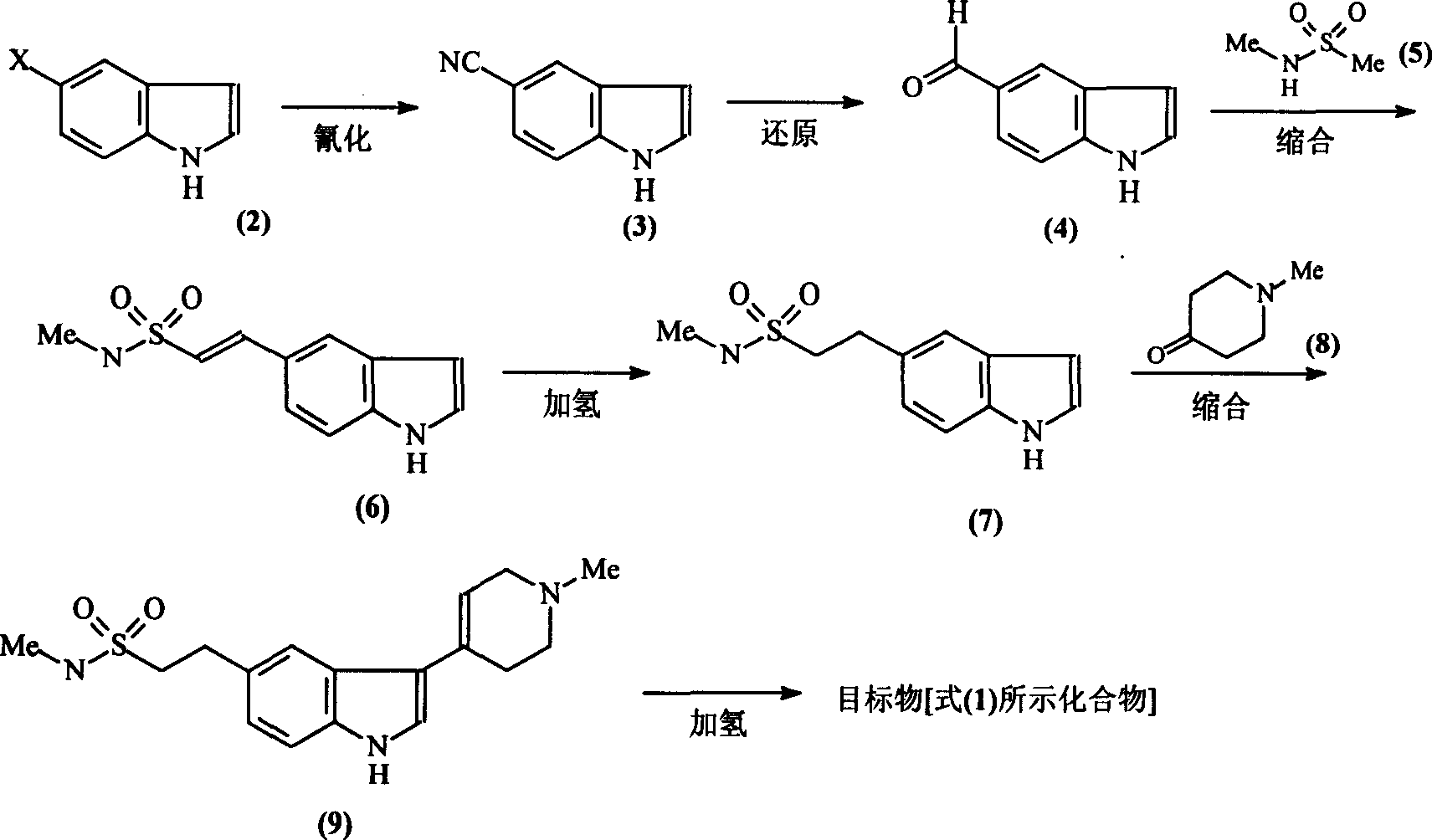
[0017] (!) Synthesis of 5-cyanindole [compound (3)]:
[0018]
[0019] A mixture of 4g (0.021mol) of compound (2), 3g (0.033mol) of CuCN, and 20ml of NMP (N-methylpyrrolidone) was placed in a 200W microwave oven for 30min, then the reactant was cooled to room temperature and washed with 50ml of ice water Dilute, filter. The obtained solid was washed with 3×10 ml of ammonia water, extracted with 50 ml of chloroform Soxhlet, and then recrystallized with ethanol to obtain 2.5 g of compound (3) as light brown powder with a yield of 80%. Melting point: 104-106°C.
[0020] (2) Synthesis of indole 5-carbaldehyde [compound (4)]:
[0021]
[0022] Mix 15g (0.106mol) of compound (3), 30g (0.283mol) of sodium hypophosphite, 100ml of water, 100ml of glacial acetic acid, and 200ml of pyridine, add 10g of Raney nickel while stirring, and react the mixture at 45°C for 2h, then cool. The reactant was filtered, the filtrate was extracted with 3×20ml ethyl acetate, the organic phases w...
Embodiment 2
[0048] Except changing the reaction condition of compound (4) and compound (5), other steps are identical with embodiment 1
[0049]
[0050] A solution of 1g (0.004mol) of compound (5a) in 25ml of anhydrous tetrahydrofuran was cooled to -78°C under nitrogen protection, 1.08g (0.01mol) of n-BuOK was added with stirring, and reacted at -78°C for 1 hour, A solution of 1 g (0.004 mol) of compound (4a) in 15 ml of anhydrous tetrahydrofuran was added dropwise. After the addition was complete, THF was refluxed for 12 hours. After the end, add 20ml of ethyl acetate and 20ml of saturated ammonium chloride solution respectively, separate layers, extract the aqueous phase with 10ml of ethyl acetate, combine the organic phases, and wash with water. After washing with saturated brine, it was dried and evaporated to remove the solvent. The residue was separated by column chromatography, mobile phase: ethyl acetate:petroleum ether=1:2. The solvent was distilled off to obtain 0.54 g of ...
Embodiment 3
[0053] Except changing the reaction condition of compound (4) and compound (5), other steps are identical with embodiment 1
[0054]
[0055] Cool the solution of 3ml (0.022mol) of diisopropylamine and 16ml of anhydrous tetrahydrofuran to -78°C, add dropwise 8ml of 1.6M n-hexane solution of butyllithium (0.01mol) under the protection of nitrogen, and stir. After the temperature naturally rose to 0°C, the reactant was cooled to -78°C again, and a solution of 0.5g (0.005mol) of compound (5) in 16ml of anhydrous tetrahydrofuran was added dropwise. After the dropwise addition was completed, the reactant slowly rose to -30°C. React for 1 hour. Then the reactant was cooled to -78°C, and a solution of 0.8 (0.003 mol) compound (4a) in 8 ml of anhydrous tetrahydrofuran was added dropwise. After the addition was complete, the reaction was carried out at room temperature for 12 hours. After the end, cool the reactant to 0°C, add 50ml of 5% HCl to stir, stand to separate the layers, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com