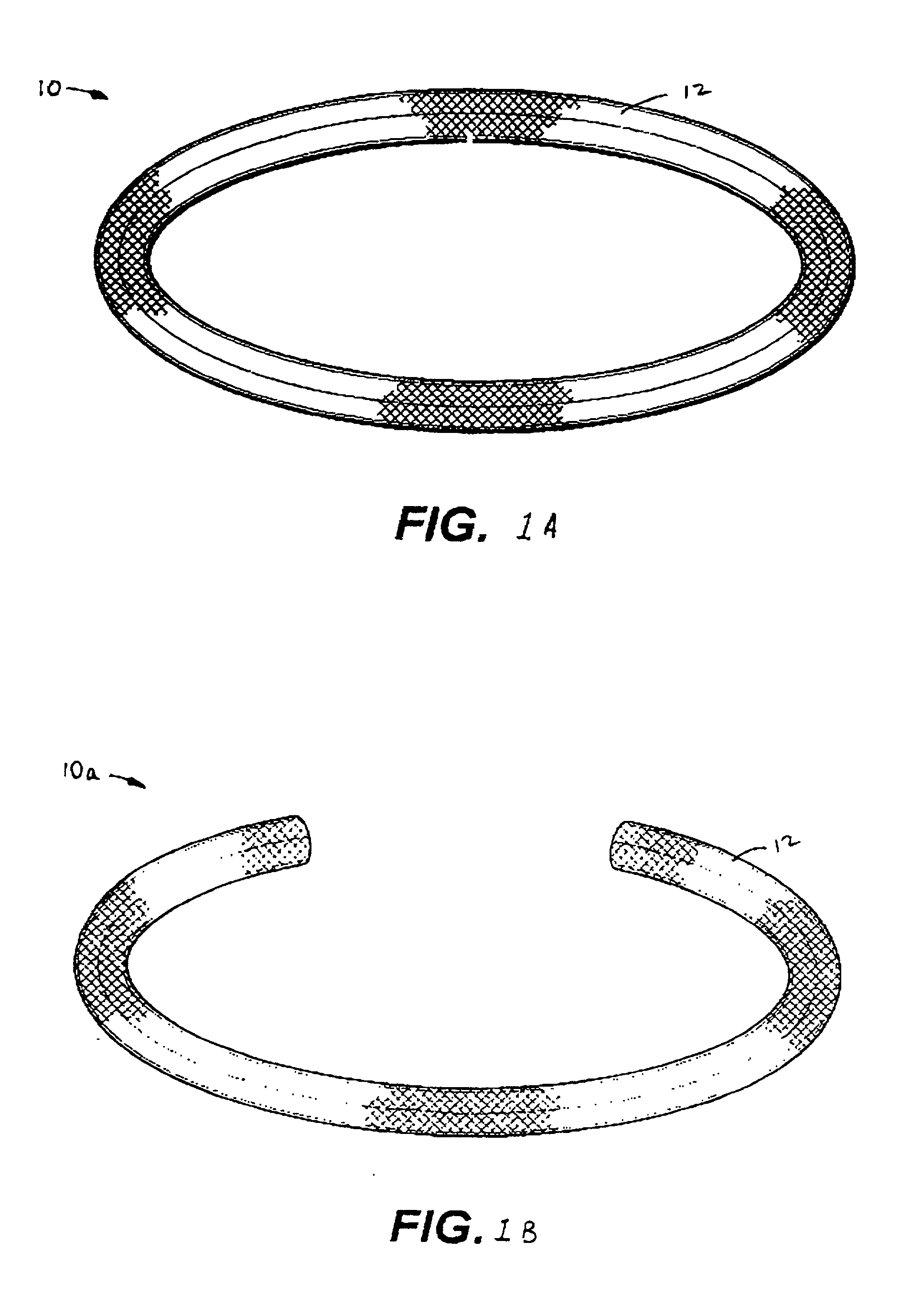
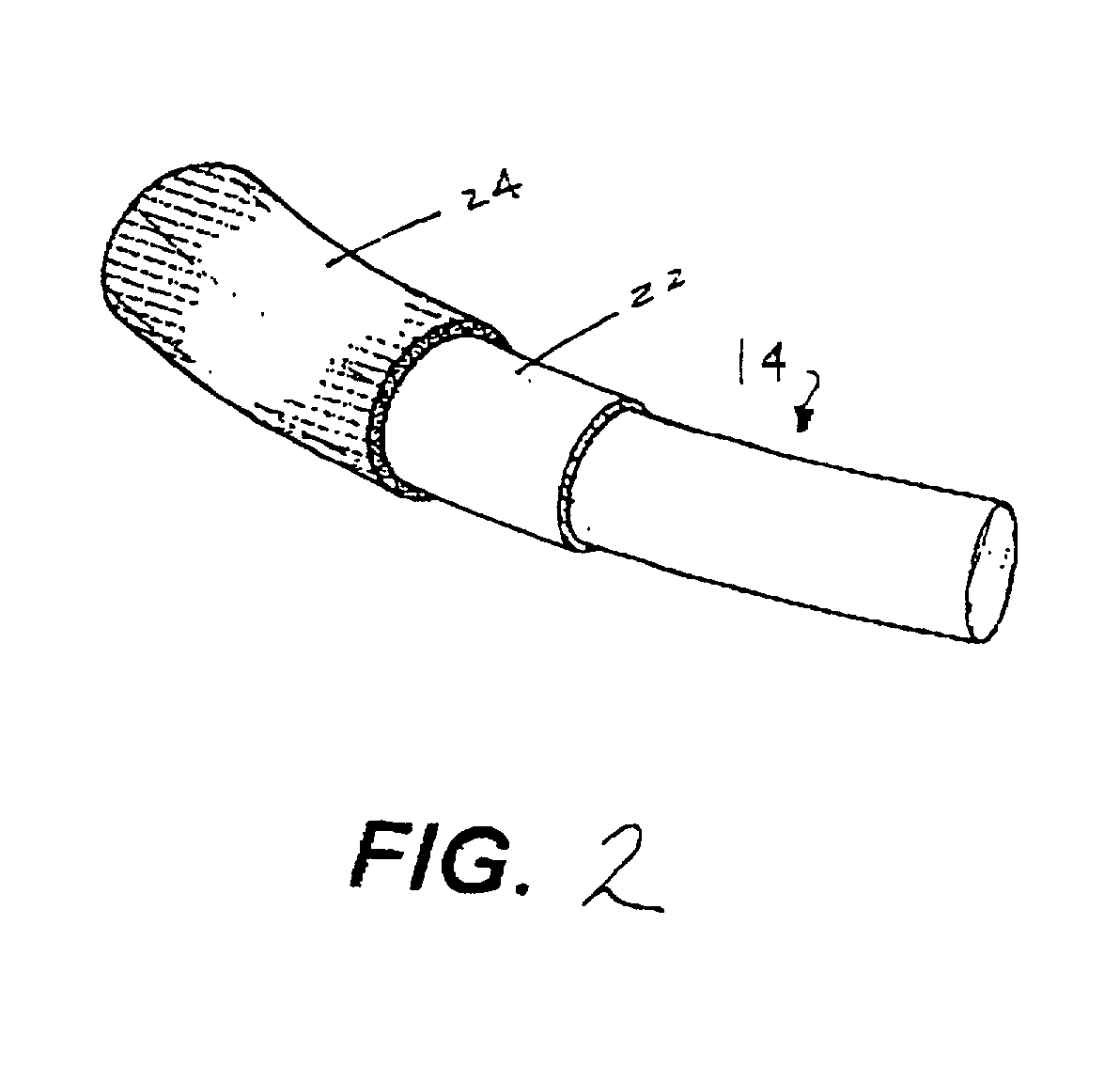
Antimicrobial annuloplasty ring having a biodegradable insert
a technology of annuloplasty ring and biodegradable insert, which is applied in the field of anti-bacterial annuloplasty ring having a biodegradable insert, can solve the problems of human heart valve deformation or other damage, surgical correction, and wires that are somewhat stiff but resiliently deformed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Antimicrobial Solutions
[0050] A process was performed essentially in accordance with U.S. Pat. No. 5,624,704. Briefly, in a dark glass bottle, 40 ml of methanol was heated to about 45 deg.C. on a magnetic stirrer-hot plate and 0.2 g of sodium hydroxide was dissolved therein. Heating was removed and 5 g minocycline, 8 g rifampin, and 80 ml butyl acetate, were dispersed in the solvent. 20 ml aliquots of the mixture were transferred to glass beakers containing either a pre-weighed polyethylene terepthalate sewing cuff assembly (Carbomedics Prosthetic Heart Valve, CPHV.TM., 27 Mitral) or a polytetrafluoroethylene felt ring (27 Mitral), prepared at Sulzer Carbomedics Inc. (Austin, Tex.). The samples were incubated in the antimicrobial solutions for approximately 1.5 hours at about 45 deg.C. After incubation, the treated cuffs and felts were removed from the antimicrobial solution and air-dried overnight. After drying, each sample was weighed and transferred to a sterile ba...
example 2
Antimicrobial Agent Incorporation
[0053] Antibiotic incorporation into or onto the sewing cuff and felt samples was monitored by determining the change in mass before and after incorporation, and also by high-performance liquid chromatography (HPLC) analysis. For HPLC analysis, the dried samples were placed in glass beakers containing 30 ml of methanol, and this extraction solution was sonicated for about 30 minutes. The supernatant was poured into a dark glass jar. The extraction process was repeated up to three times and the extracts were combined and analyzed by HPLC using a Beckman Nouveau Gold HPLC system (Beckman Instruments; Fullerton, Calif.). The samples were diluted as necessary and injected in 0.1 M sodium phosphate, pH 3.2. 25 ul of each sample was injected by an auto sampler into the HPLC system. Sample separation was achieved using acetonitrile:water (4:6 v / v), with a flow rate of 1 ml / min, using a Water's C18 Nova Pak 60A, 4 um, 3.9.times.150 mm column, maintained at a...
example 3
Release Profiles for Rifampin and Minocycline
[0056] The kinetics of antibiotic release from the treated samples was evaluated by incubating the samples in 15 ml phosphate buffered saline (PBS) at about 37 deg.C. for 30 days. For about the first hour, the samples were gently agitated in PBS at room temperature in 15 ml PBS. This PBS solution was removed and frozen until further analysis. Fresh PBS was added and the samples were placed in a 37 deg.C. incubator.
[0057] The PBS was thereafter replaced at 1, 2, 4, 5, 7, 11, 15, 21, 25, and 30 days, and each aliquot was frozen until subsequent analysis by HPLC. The release profiles for rifampin and minocycline are summarized below in Tables 3 and 4, respectively.
3TABLE 3 RELEASE OF RIFAMPIN (mg) OVER 30 DAYS Cuff Felt Day Cuff (methanol Felt (methanol collected (U.S. 5,624,704) only) (U.S. 5,624,704) only) 0 14.0 14.6 10.6 5.85 1 22.1 19.9 12.7 13.5 2 13.4 13.1 2.16 5.63 4 5.37 10.1 0.66 1.31 5 0.72 2.45 0.25 0.12 7 0.29 0.59 0.16 0.02 11 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com