Patents
Literature
34results about How to "Minimize compression" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
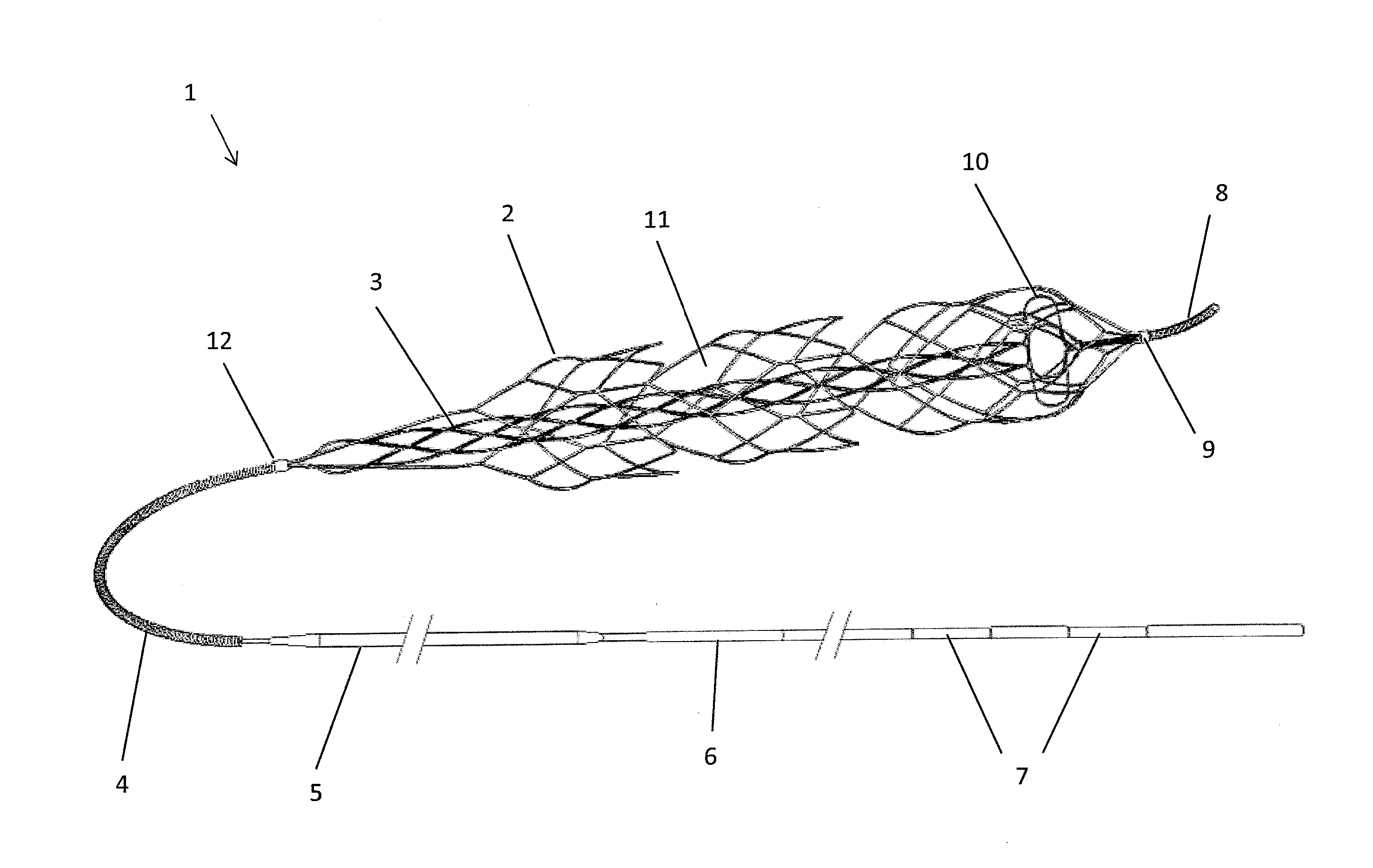
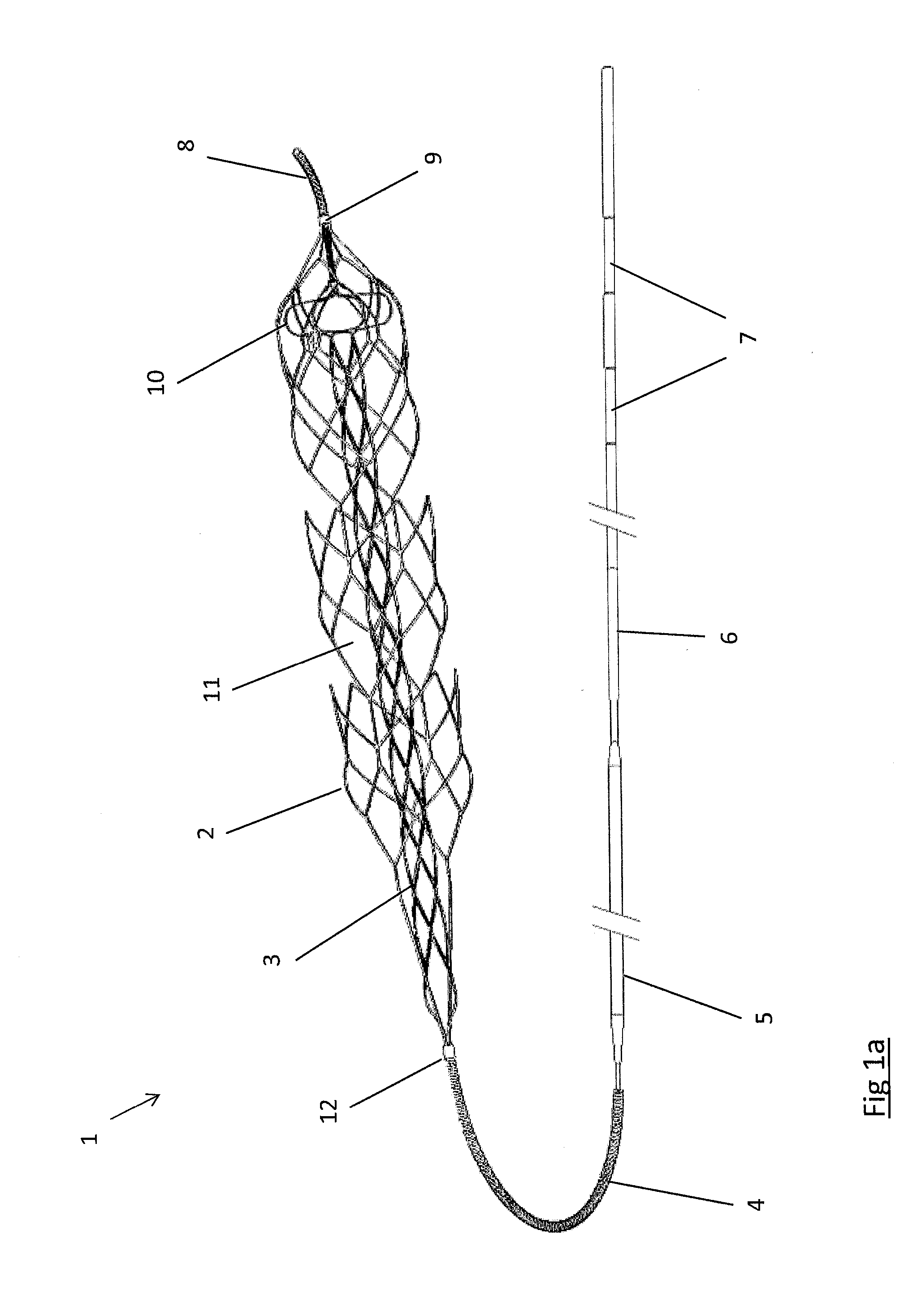
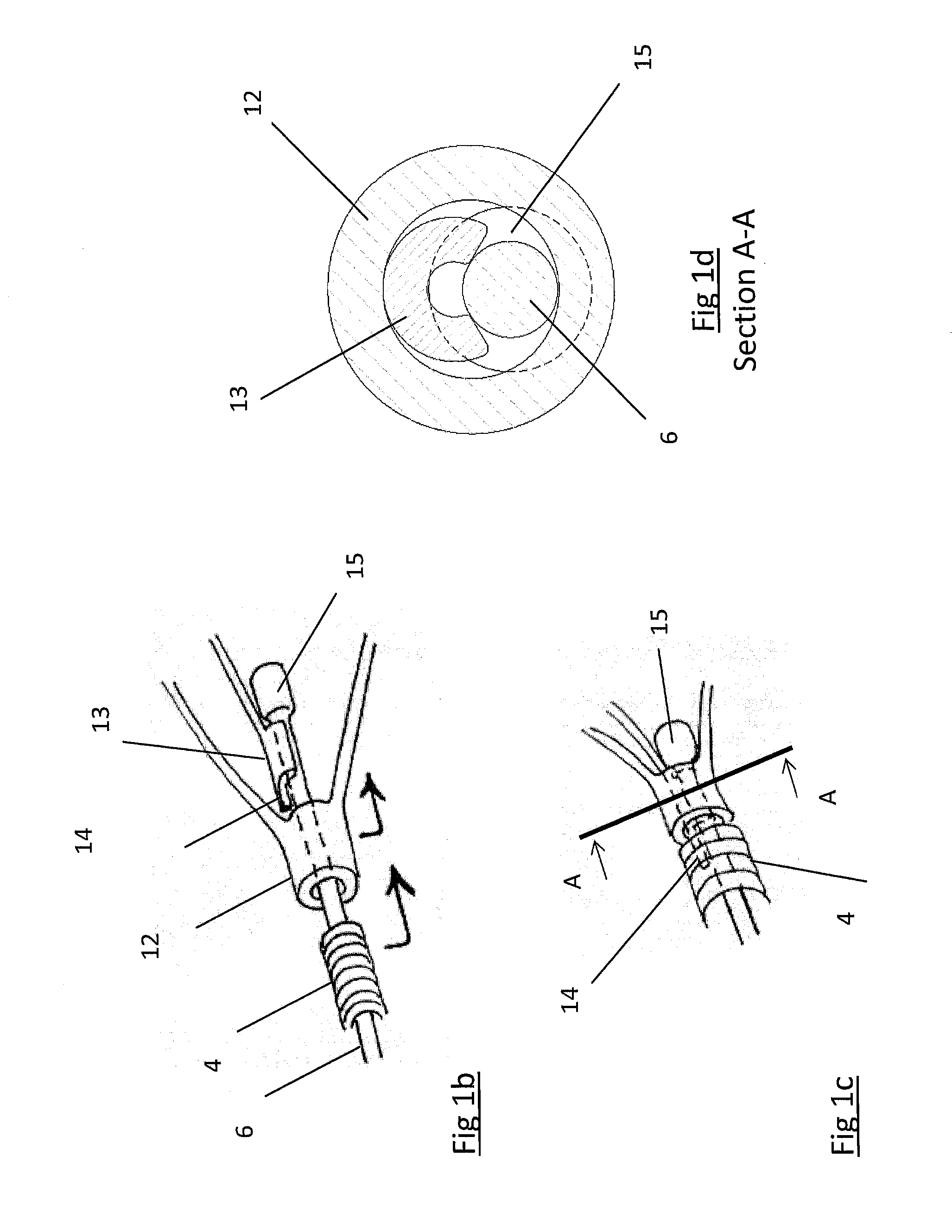
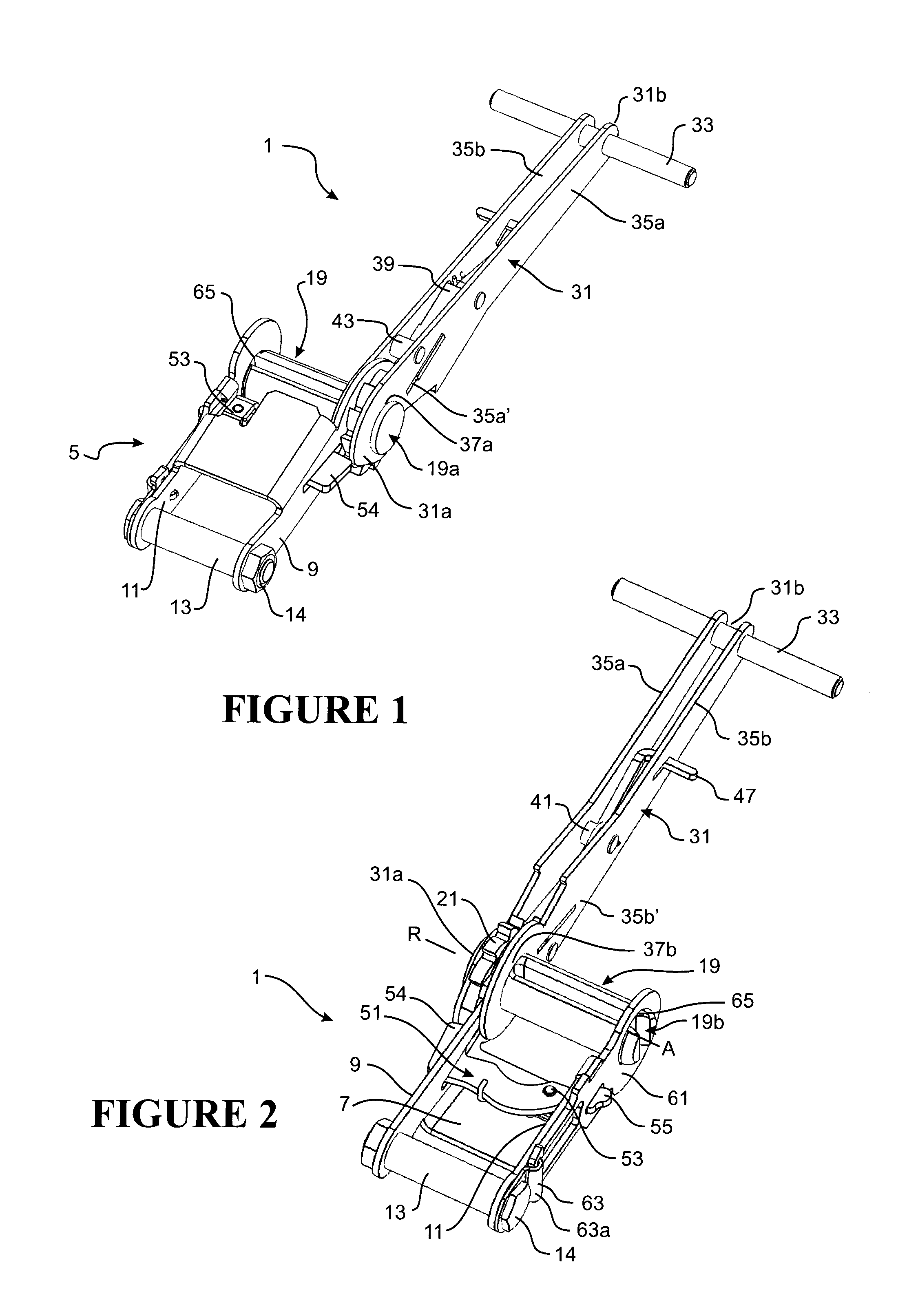
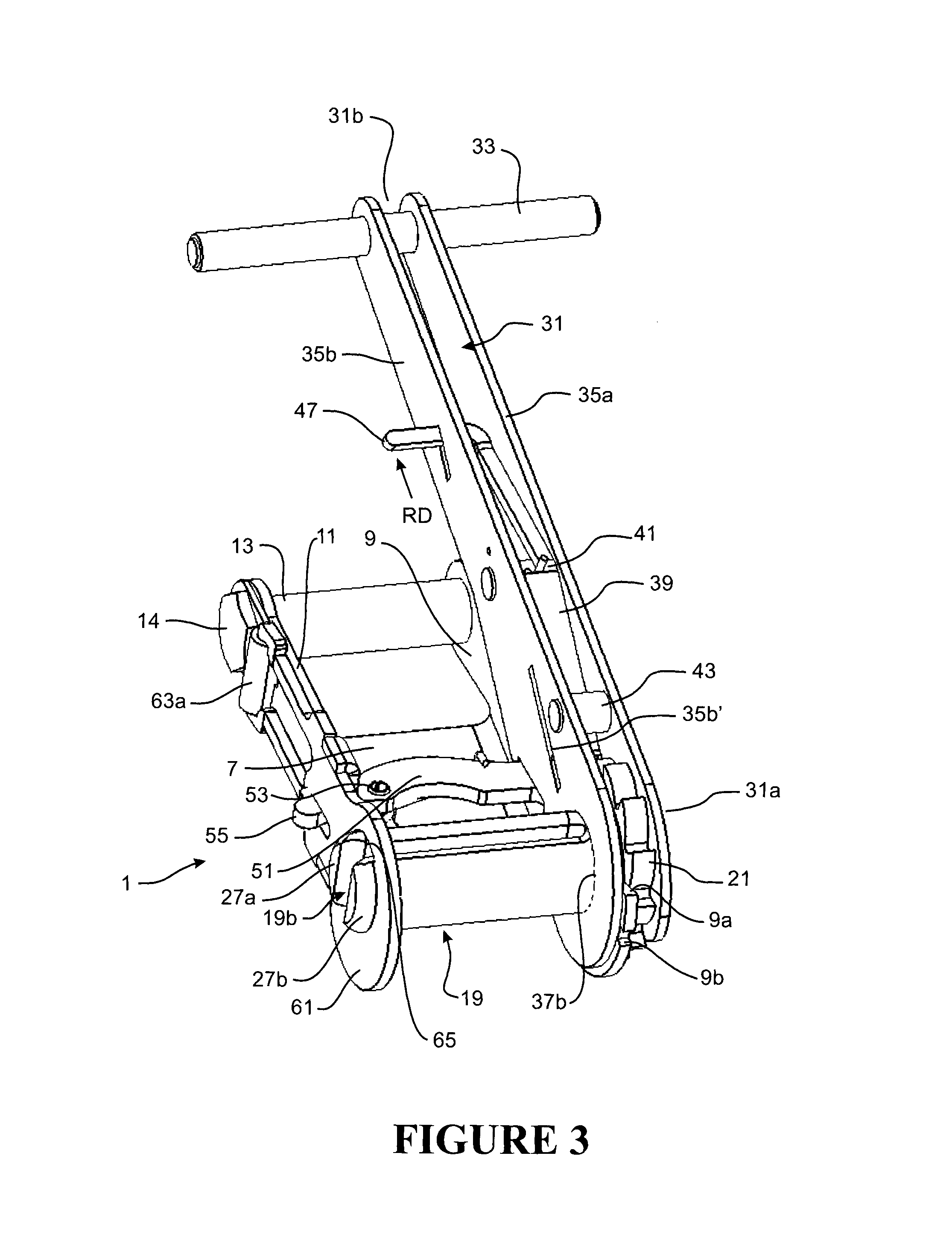
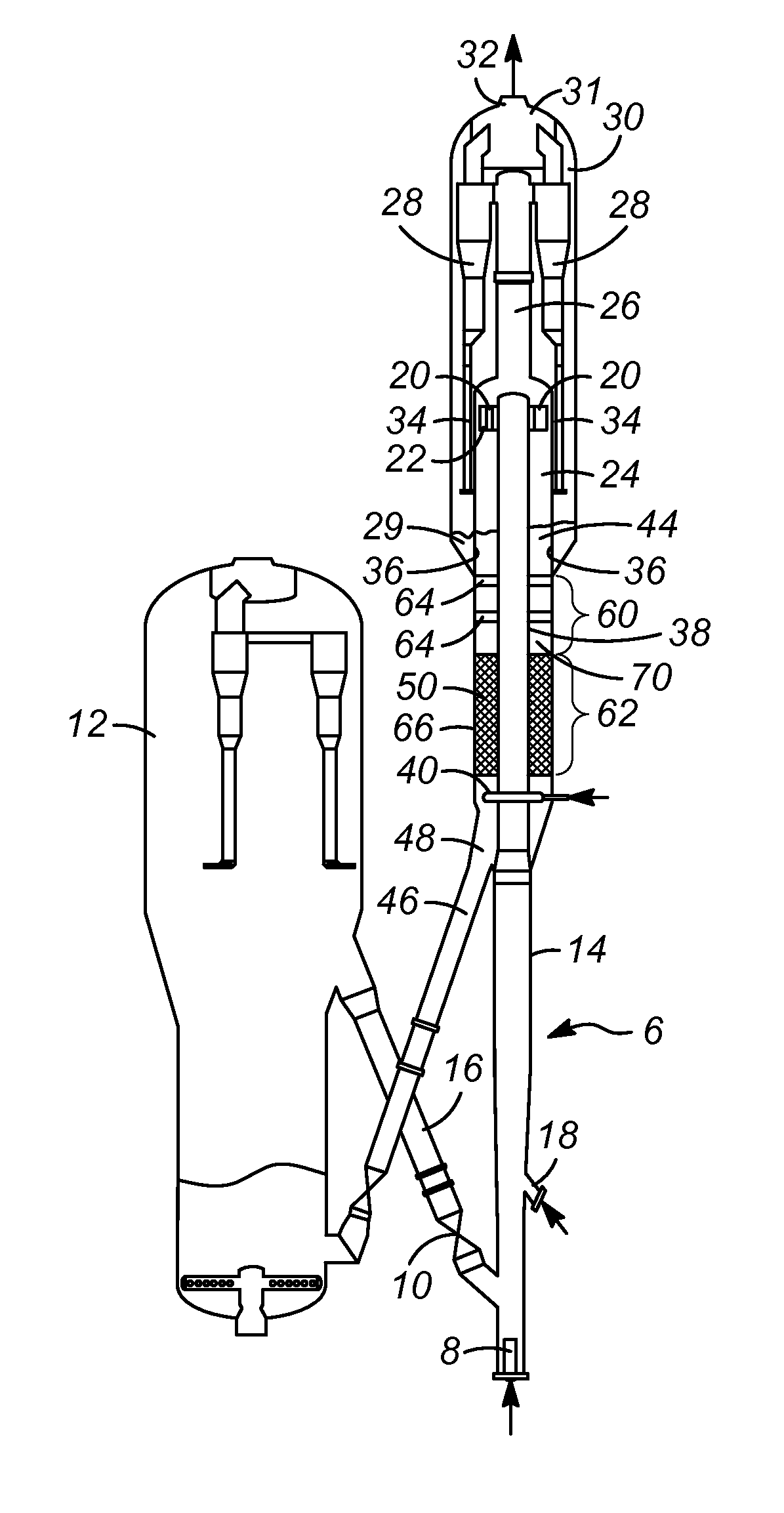
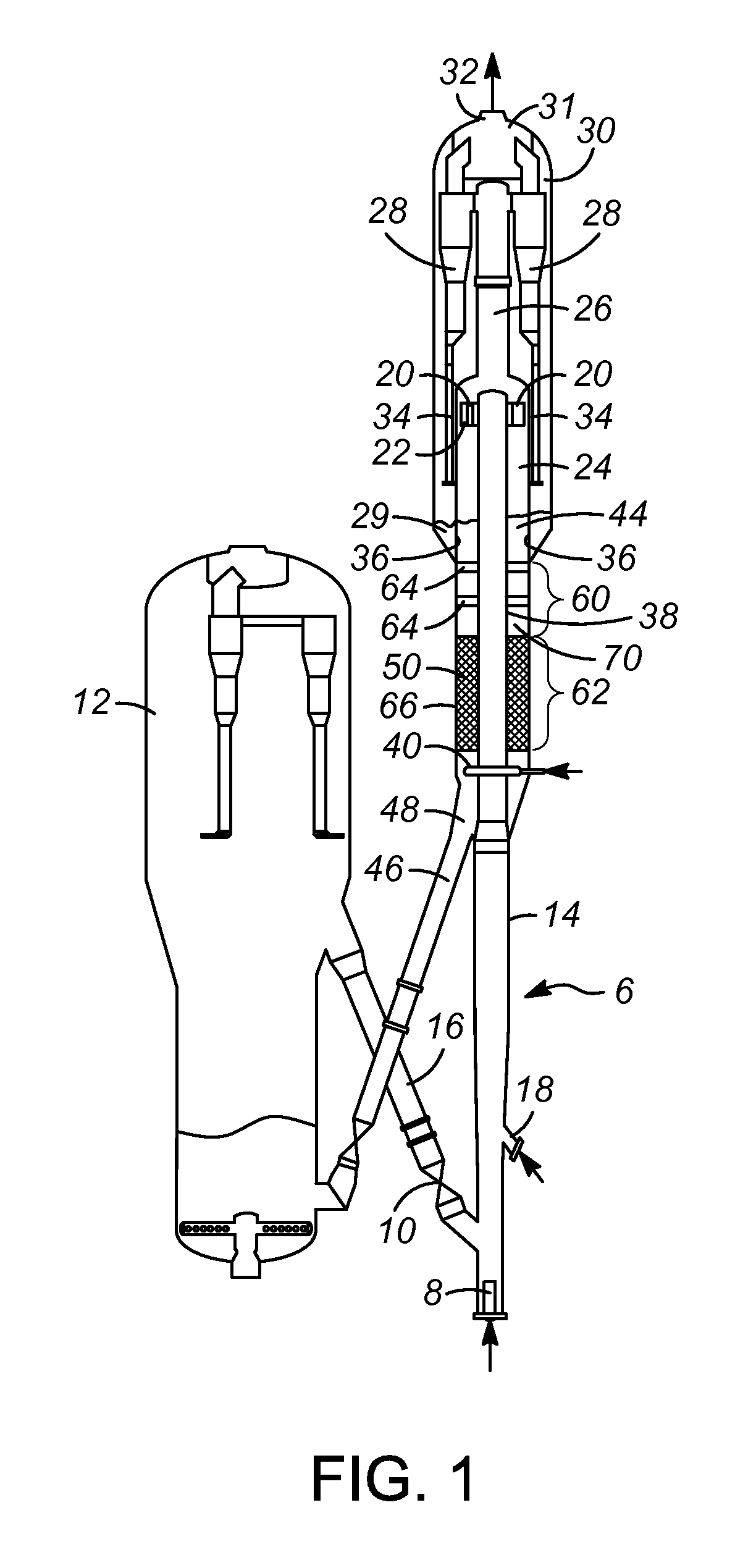
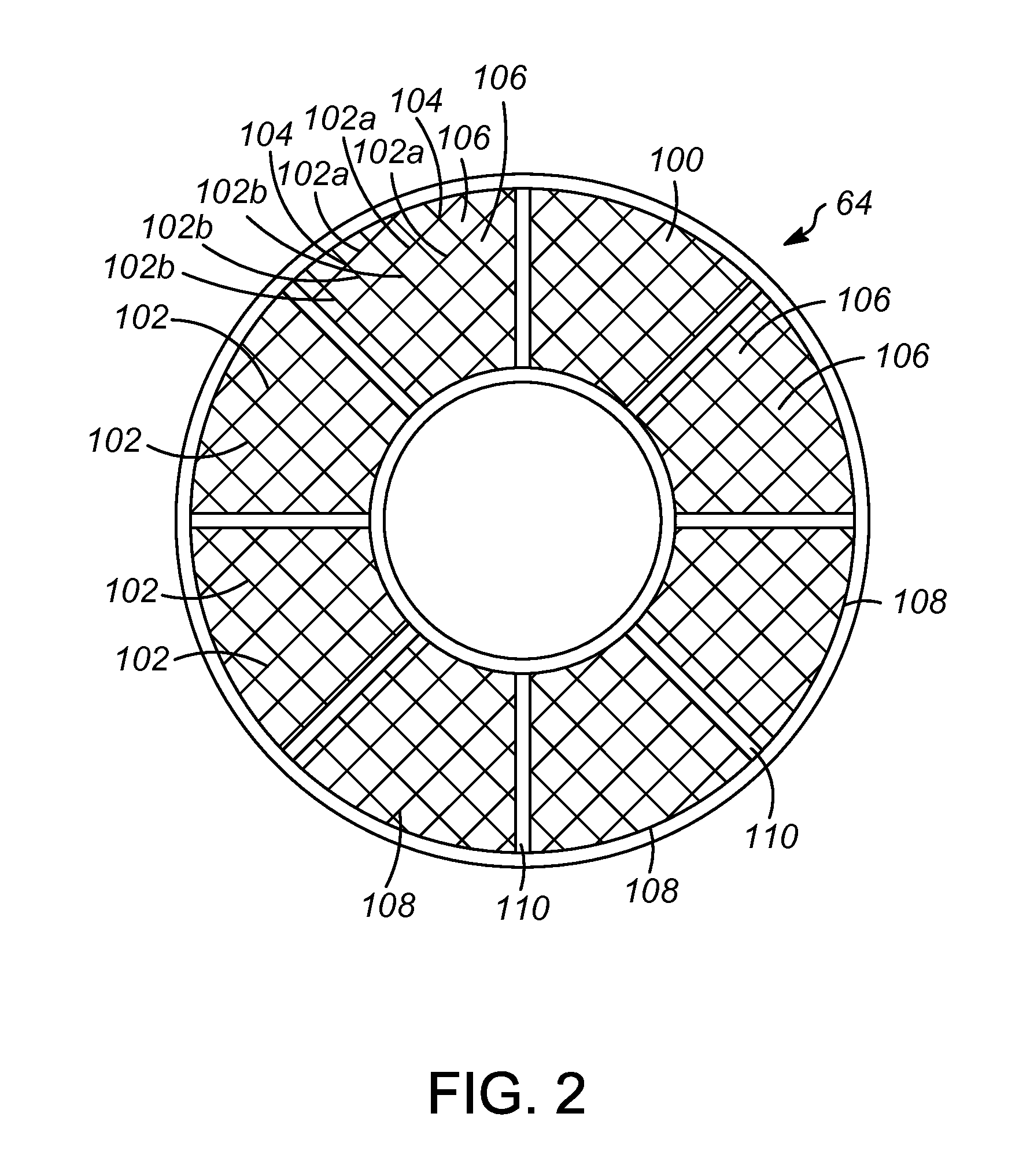
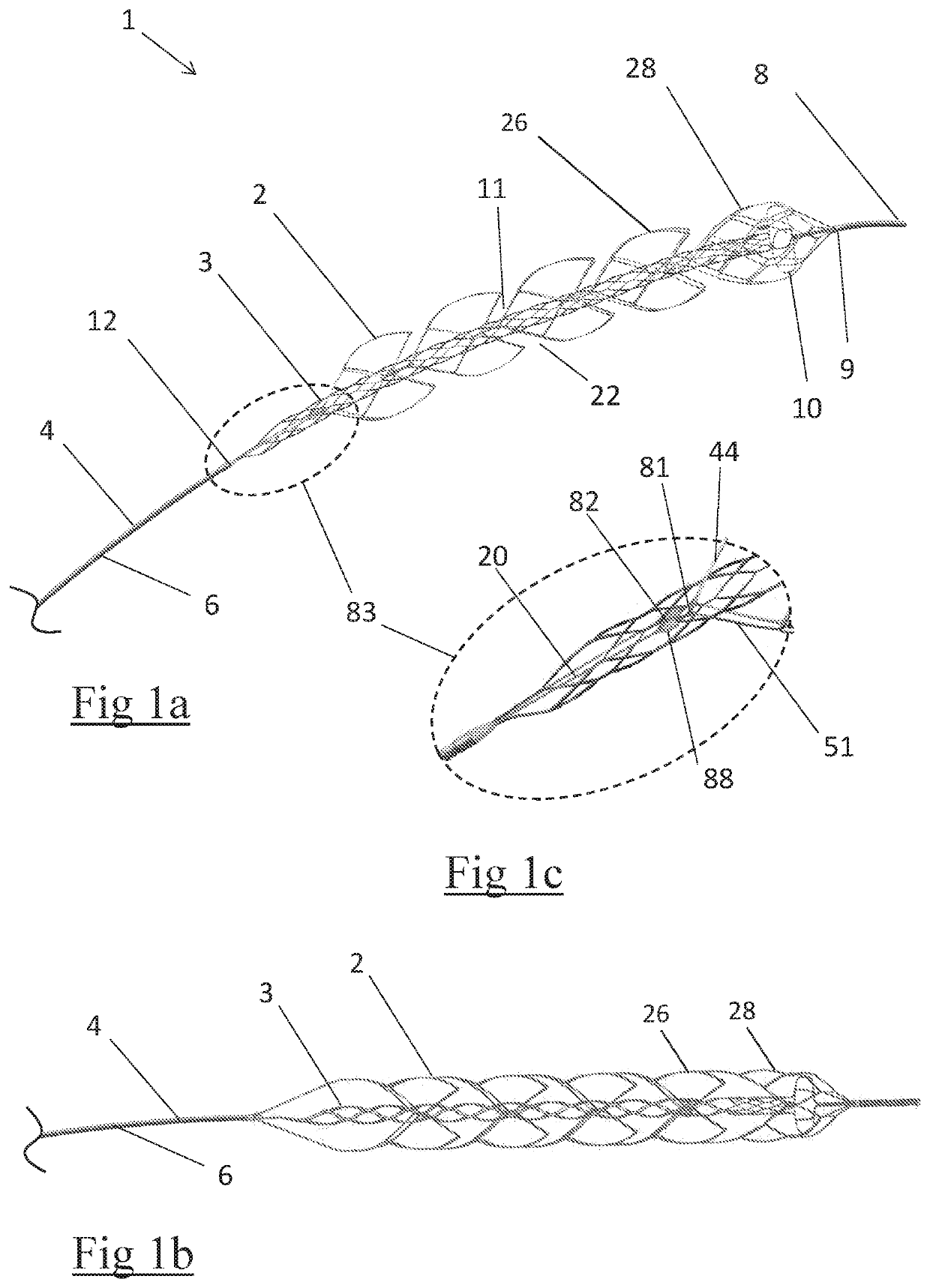
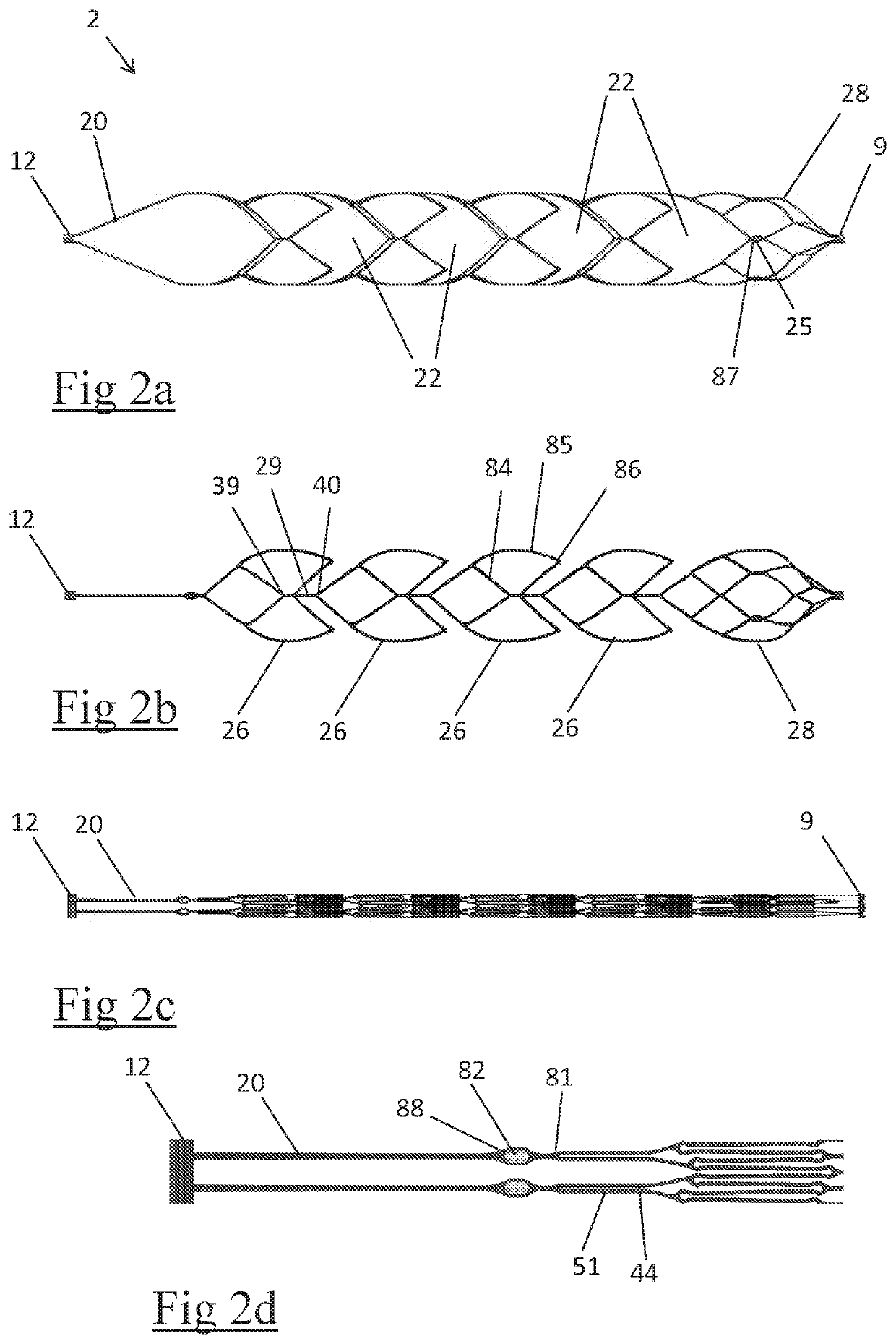
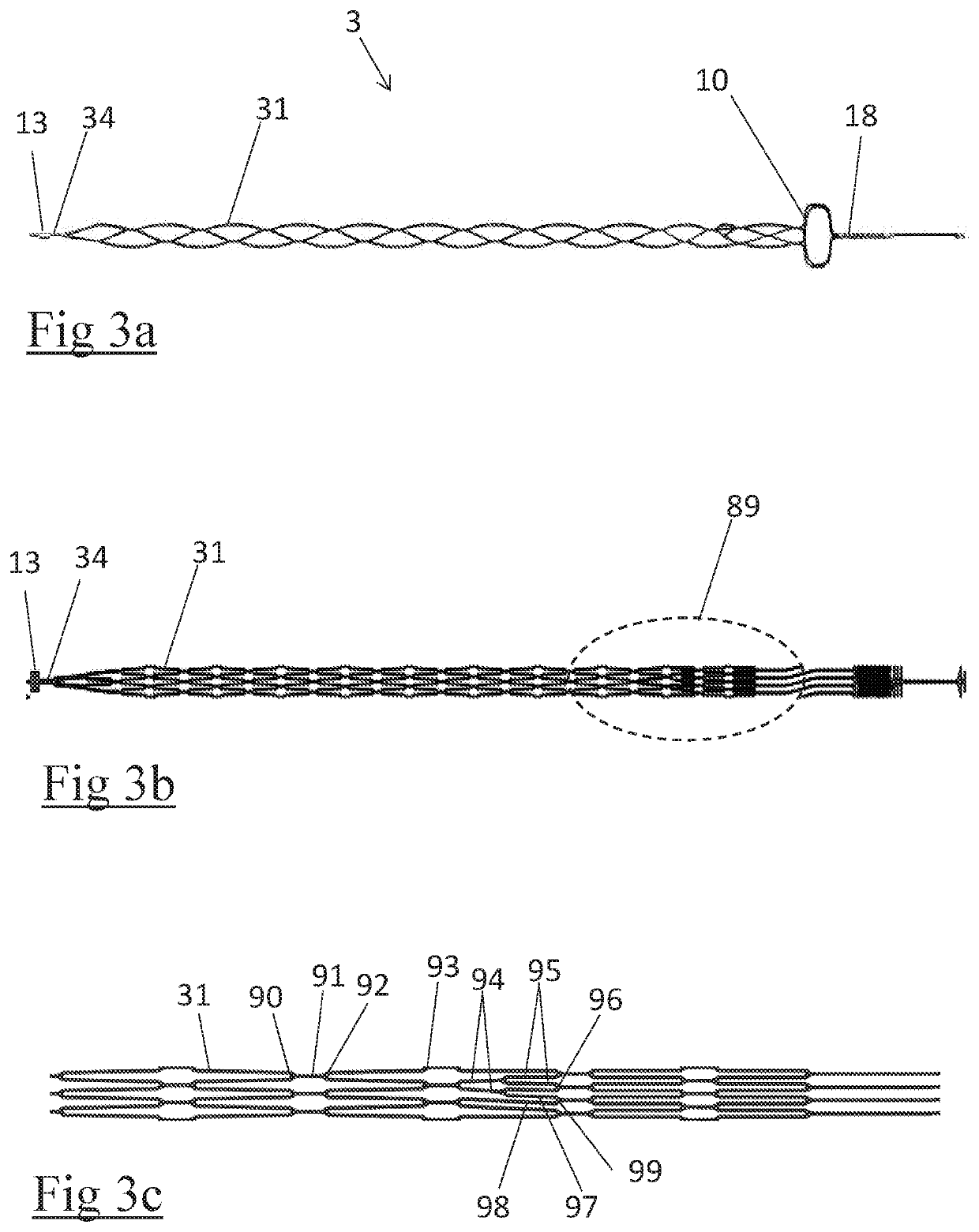
Clot retrieval device for removing occlusive clot from a blood vessel
ActiveUS20140371779A1Reduce radial forceMinimize compressionDilatorsExcision instrumentsBiomedical engineeringBlood vessel
Owner:NEURAVI
Clot retrieval device for removing occlusive clot from a blood vessel
ActiveUS20150164523A1Prevent distal egressReduce radial forceDilatorsExcision instrumentsDistal portionBlood vessel spasm
Owner:NEURAVI
Cmp pad conditioners and associated methods
InactiveUS20080153398A1Reduce degree of compressionMinimize compressionGrinding drivesBelt grinding machinesBiomedical engineering
A method of reducing a degree of compression of a CMP pad during conditioning of the CMP pad comprises engaging the CMP pad with at least one superhard cutting element, the cutting element including a cutting face, the cutting face being angled at 90 degrees or less relative to a finished surface of the CMP pad; and moving the CMP pad and the cutting element relative to one another in a direction resulting in removal of material from the CMP pad with the cutting face to thereby condition the CMP pad.
Owner:SUNG CHIEN MIN
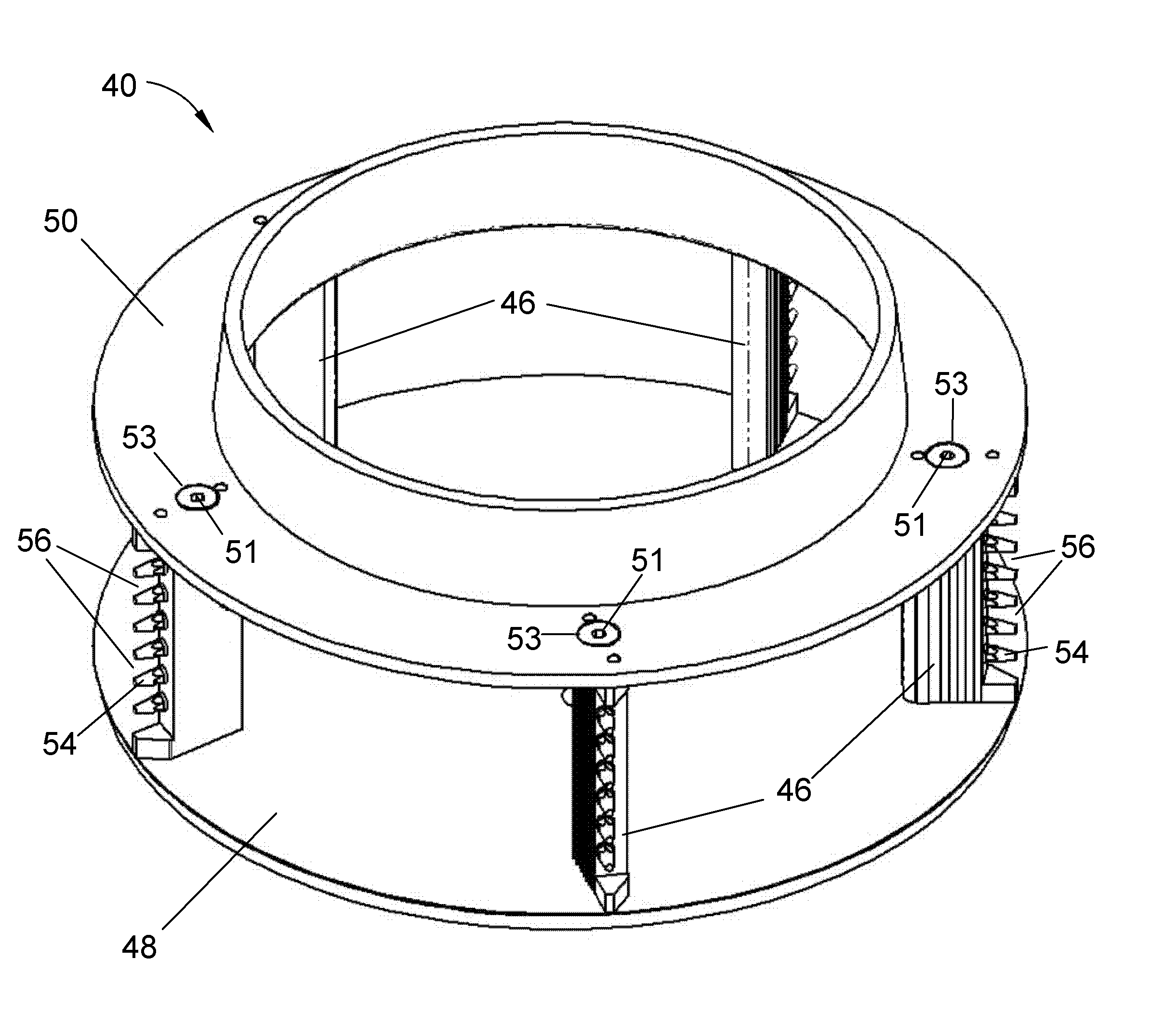
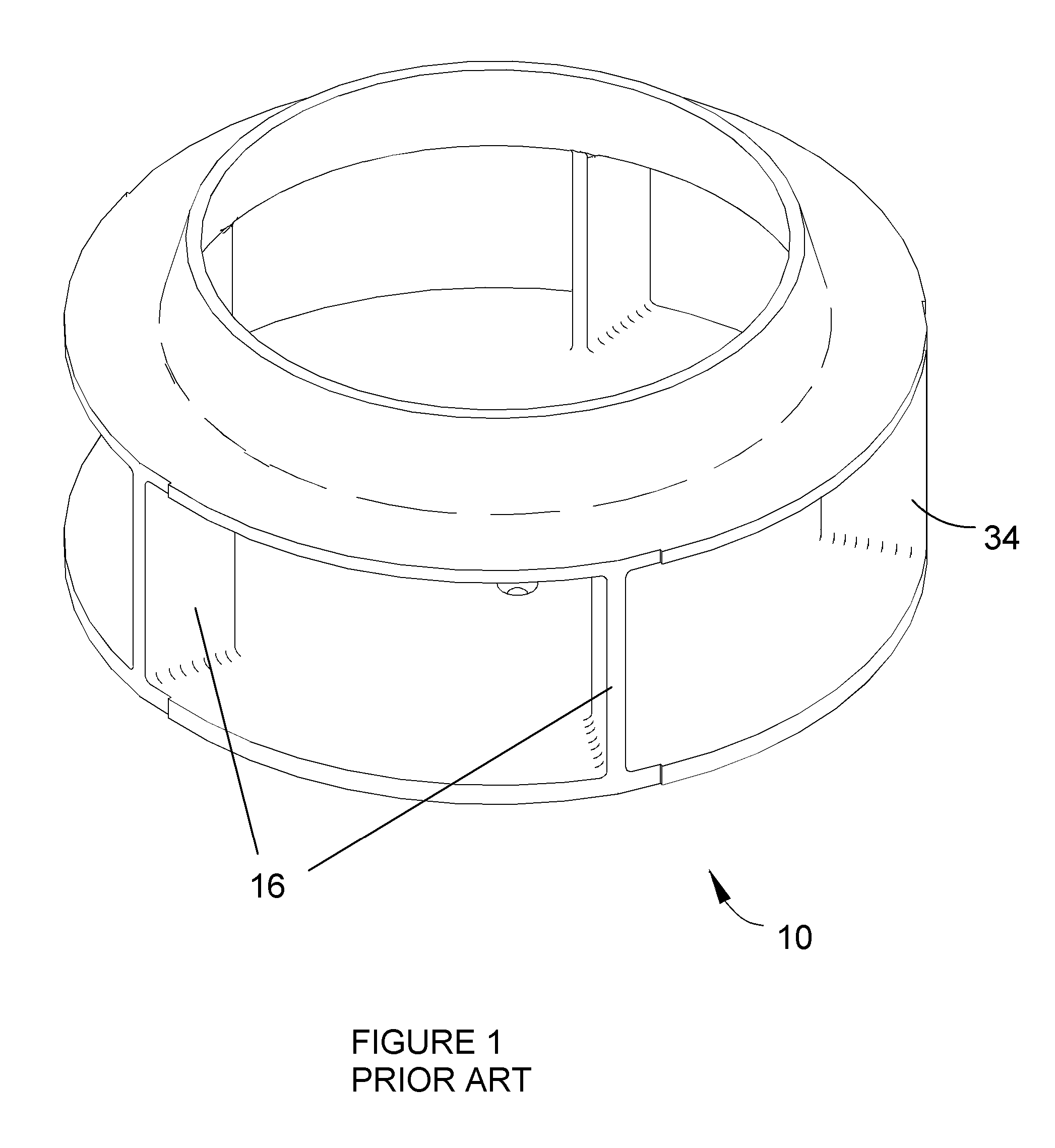
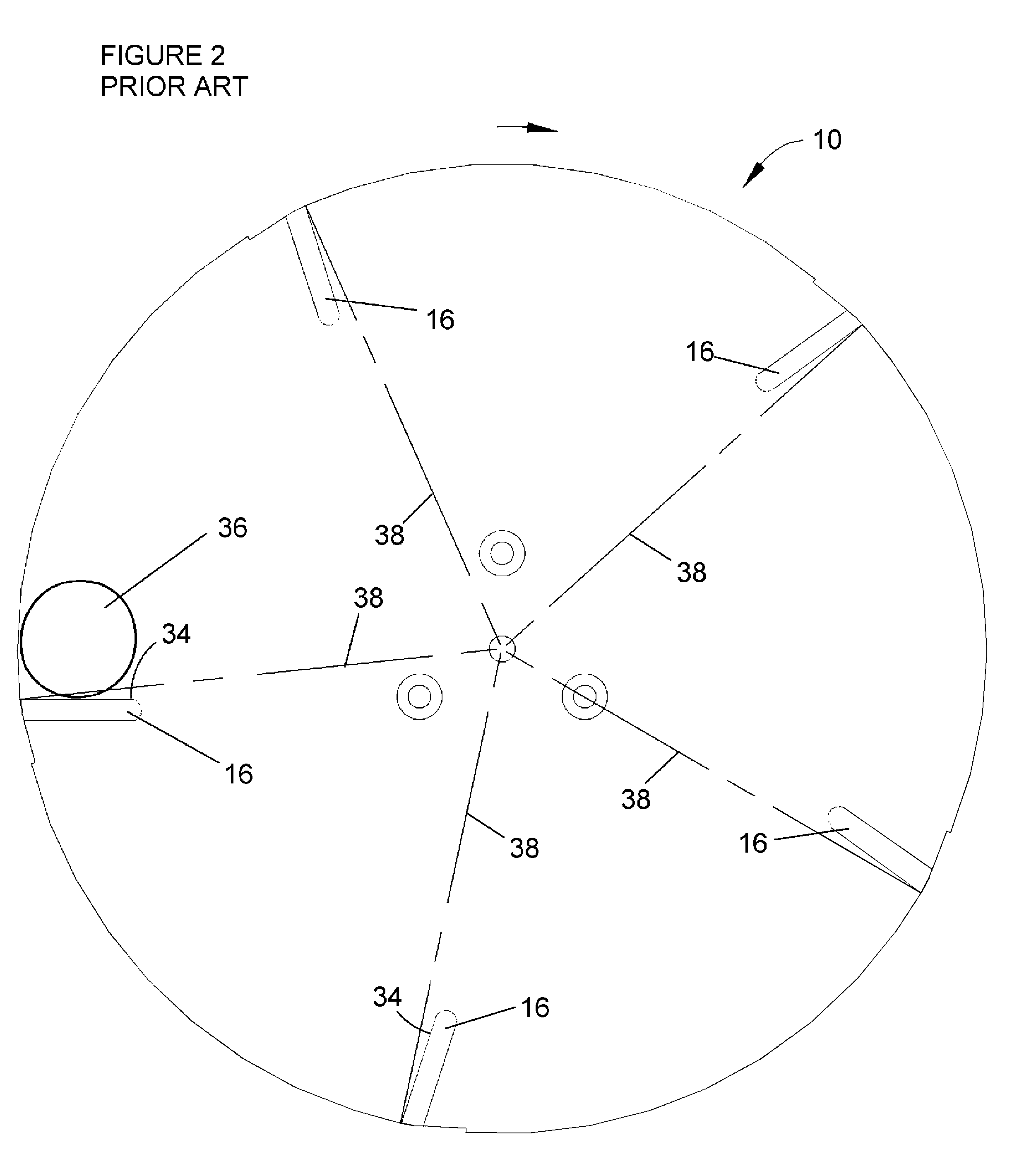
Apparatus for cutting food product
A cutting apparatus having an annular-shaped cutting head and an impeller assembly coaxially mounted for rotation within the cutting head to deliver food products radially outward toward the cutting head. The cutting head has at least one knife extending radially inward toward the impeller assembly. The knife has a cutting edge at a radially innermost extremity and a radially outer face that defines a trajectory plane for slices removed from the products by the cutting edge. The knife is clamped to the cutting head with a clamping feature that provides clearance for slices when traveling the trajectory plane of the knife.
Owner:URSCHEL LAB +1
Apparatus for cutting food product
ActiveUS20070240550A1Minimize compressionConsistent thicknessMetal working apparatusImpellerBiomedical engineering
A cutting apparatus having an annular-shaped cutting head and an impeller assembly coaxially mounted for rotation within the cutting head to deliver food products radially outward toward the cutting head. The cutting head has at least one knife extending radially inward toward the impeller assembly. The knife has a cutting edge at a radially innermost extremity and a radially outer face that defines a trajectory plane for slices removed from the products by the cutting edge. The knife is clamped to the cutting head with a clamping feature that provides clearance for slices when traveling the trajectory plane of the knife.
Owner:URSCHEL LAB +1
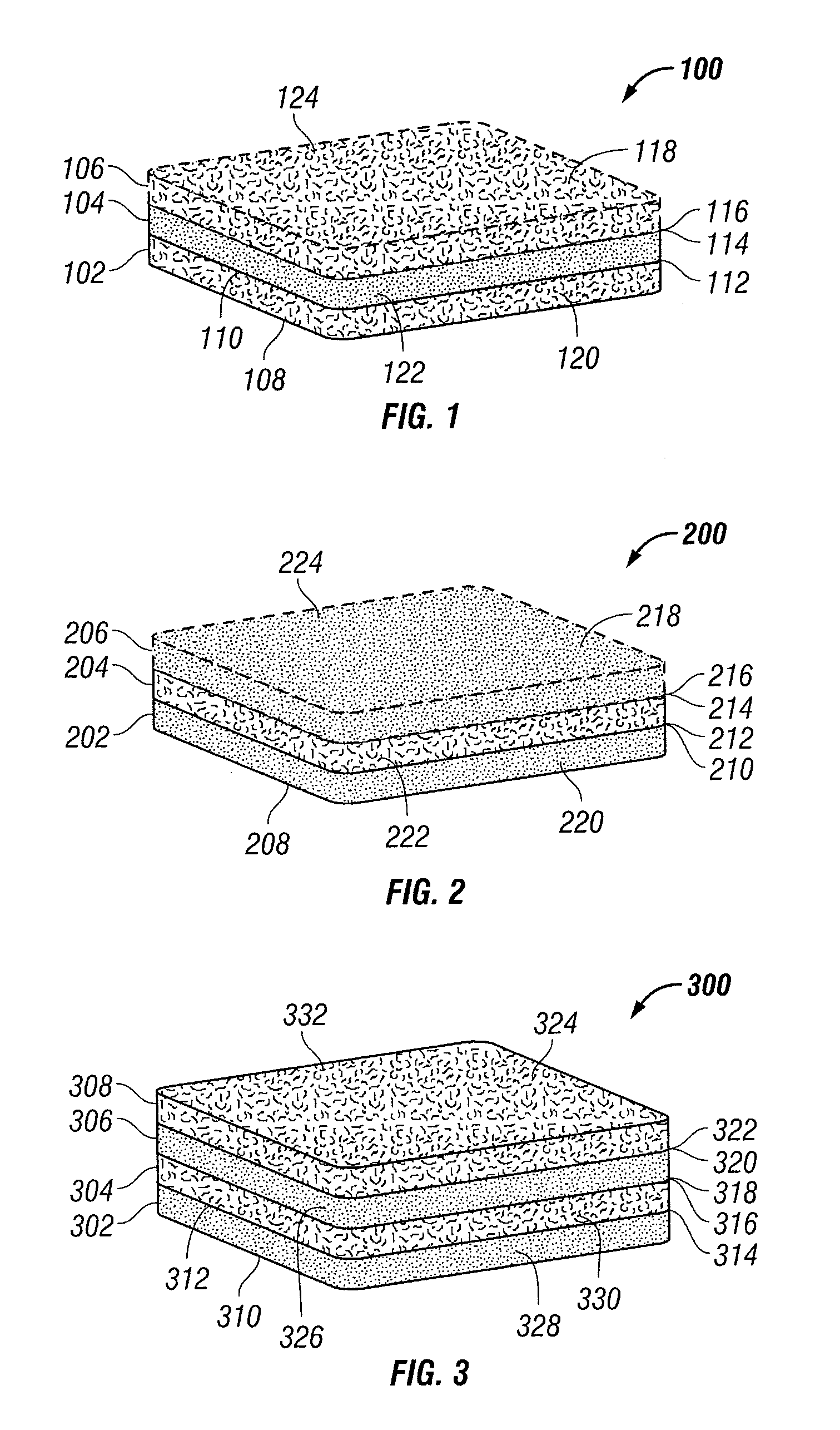
Fiber-microsphere bioresorbable composite scaffold for wound healing
ActiveUS20090198167A1Stable supportIncrease flexibilityNon-adhesive dressingsWound drainsWound healingMicrosphere
A fiber-microsphere composite scaffold including a first layer of material selected from one of a layer of bioresorbable microspheres and a layer of bioresorbable fibers; and a second layer of material selected from the other of the layer of bioresorbable microspheres and the layer of bioresorbable fibers. A fiber-microsphere composite scaffold reduced pressure tissue treatment apparatus is also included as well as methods for making fiber-microsphere composite scaffolds.
Owner:KCI LICENSING INC
Apparatus for cutting food product
ActiveUS7658133B2Minimize compressionGood precisionGrain treatmentsMetal working apparatusImpellerBiomedical engineering
Owner:FRITO LAY NORTH AMERICA INC +1
Chemical reactor system and process
InactiveUS6703530B2Large effective void fractionLower overall pressure dropOrganic compound preparationOrganic chemistry methodsReactor systemChemical reactor
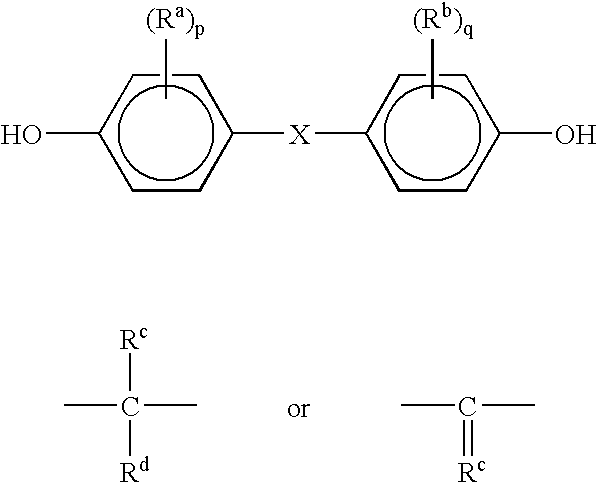
A method for producing bisphenol includes introducing a phenol and a ketone into a fixed, supported catalytic bed reactor system in a downflow mode, reacting the phenol and the ketone to form a reaction mixture, and recovering the bisphenol isomer from the reaction mixture. The preferred bisphenol isomer is bisphenol A, or p,p'-bisphenol A, produced from the reaction of phenol and acetone. The reactor for producing the bisphenol A from the reaction of phenol and acetone includes an ion exchange resin catalyst disposed in a bed and packing randomly distributed throughout the ion exchange resin catalyst to improve heat transfer efficiency and reduce compression of the catalyst bed.
Owner:SABIC GLOBAL TECH BV
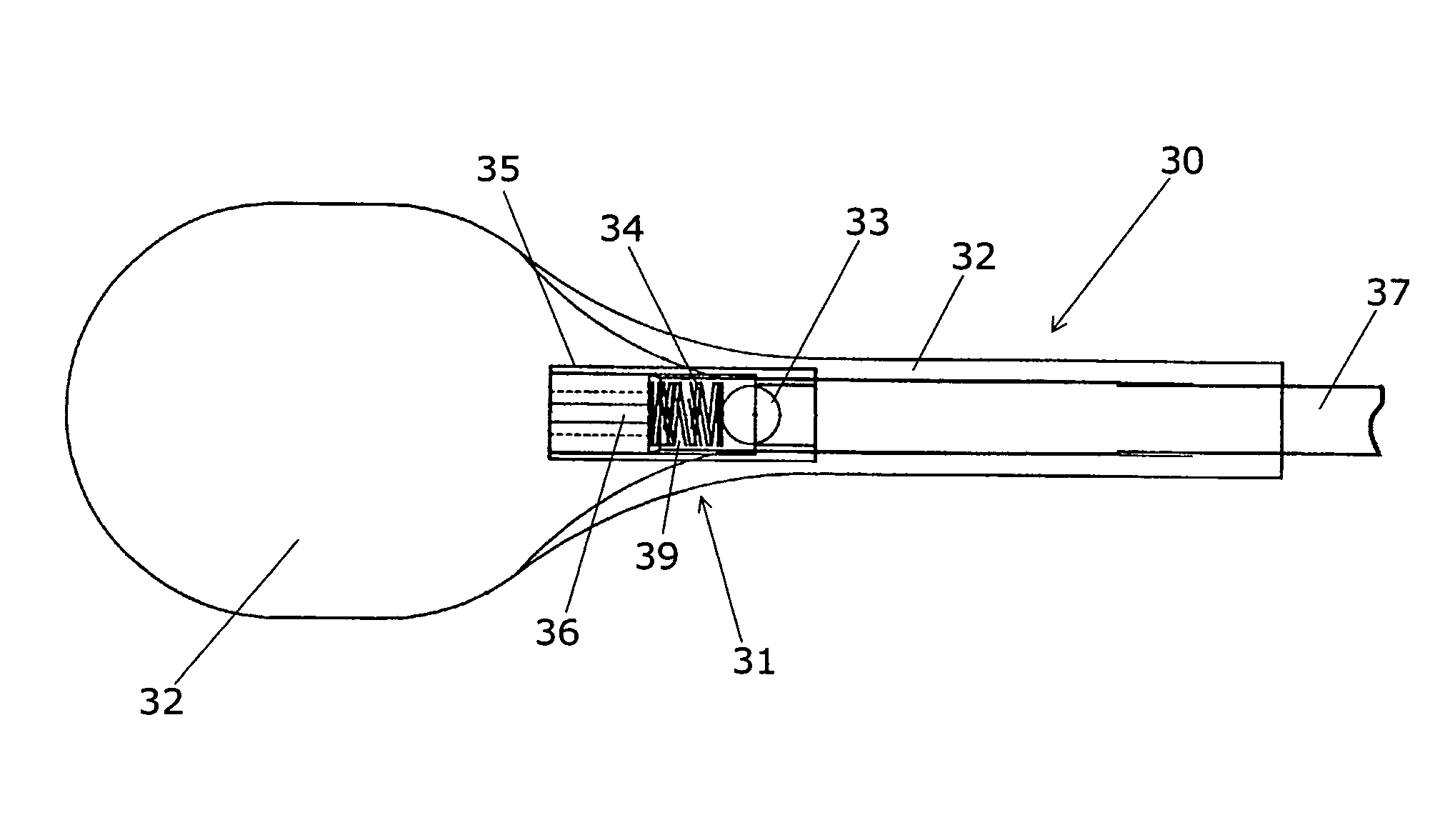
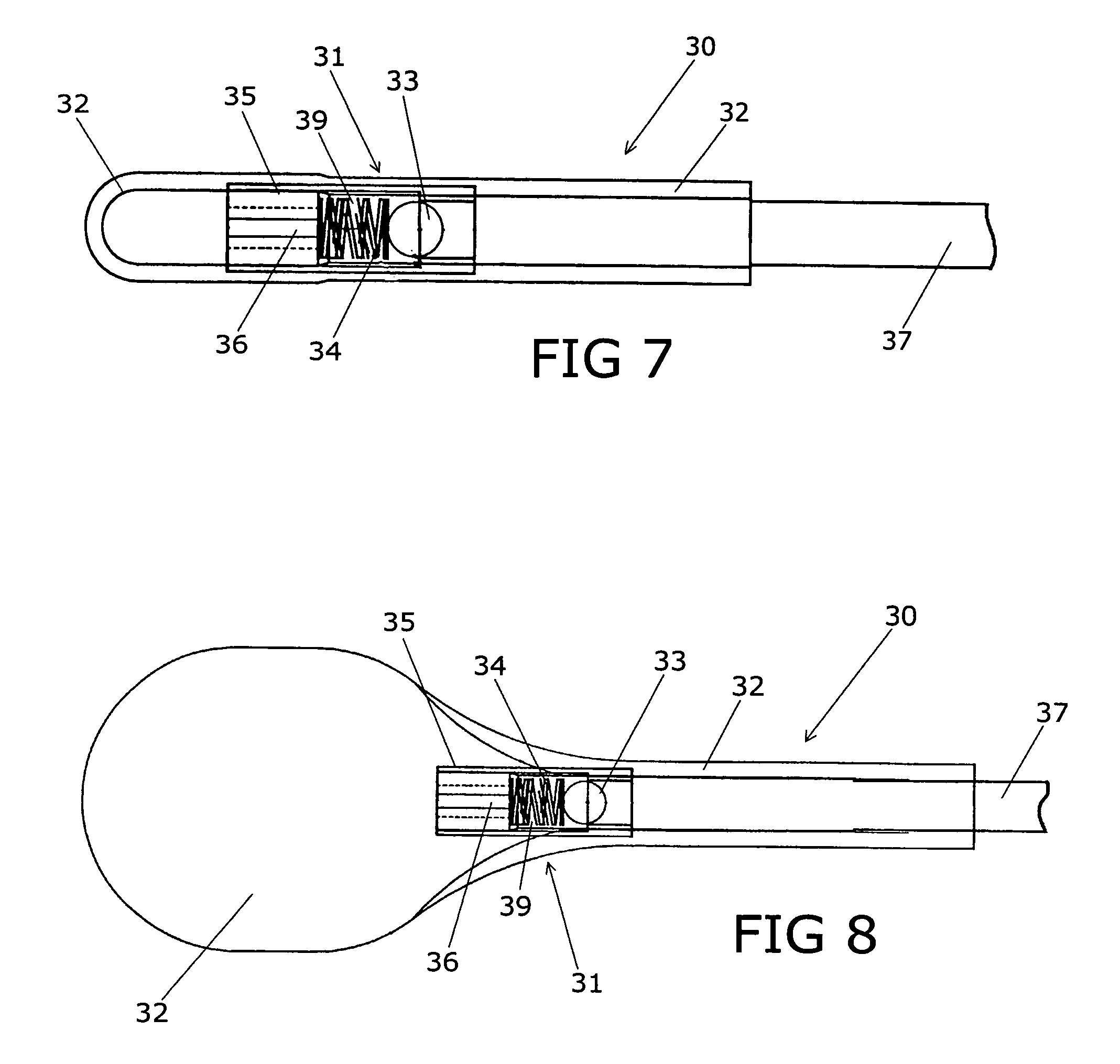
Device for treating intervertebral disc herniations
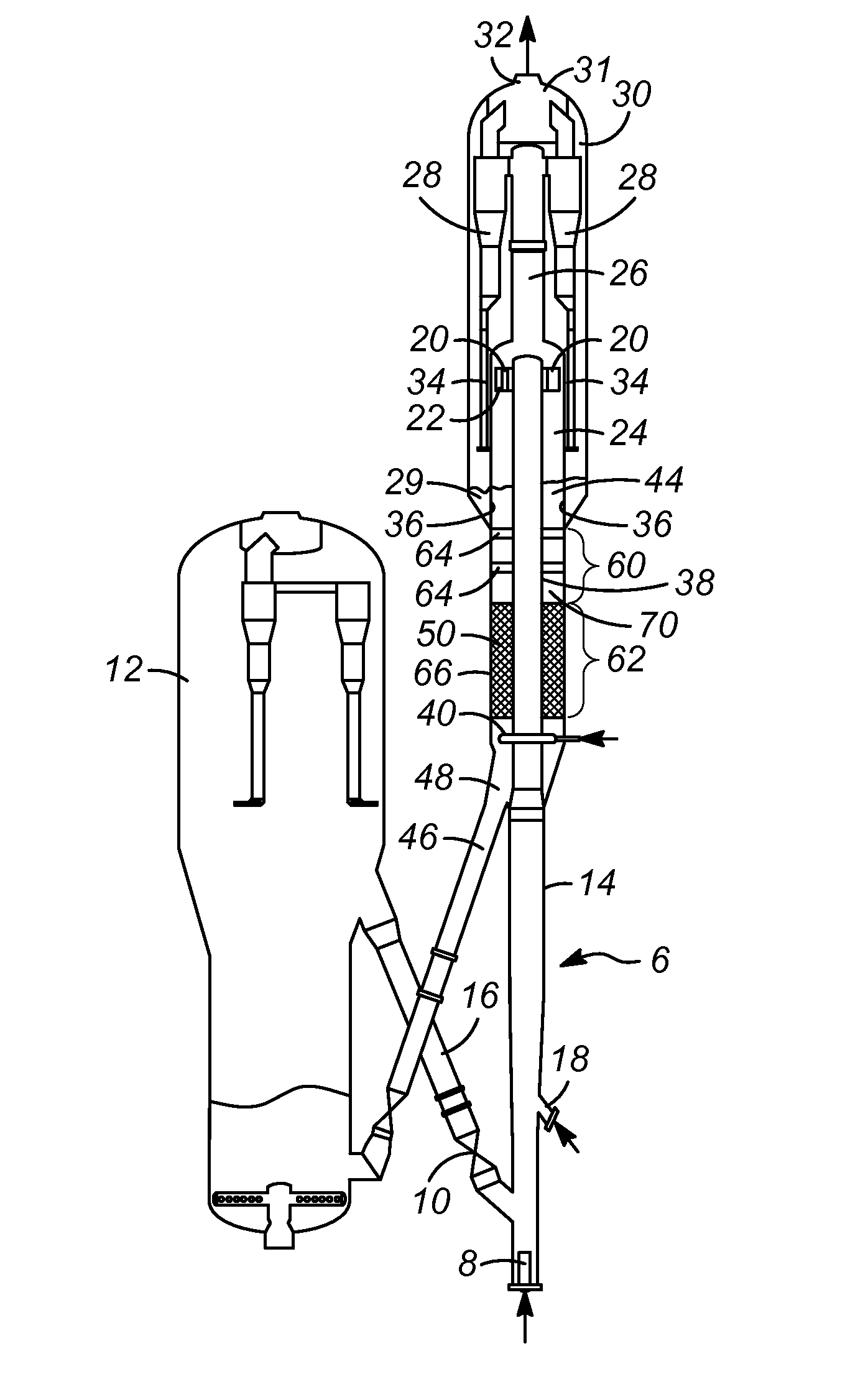
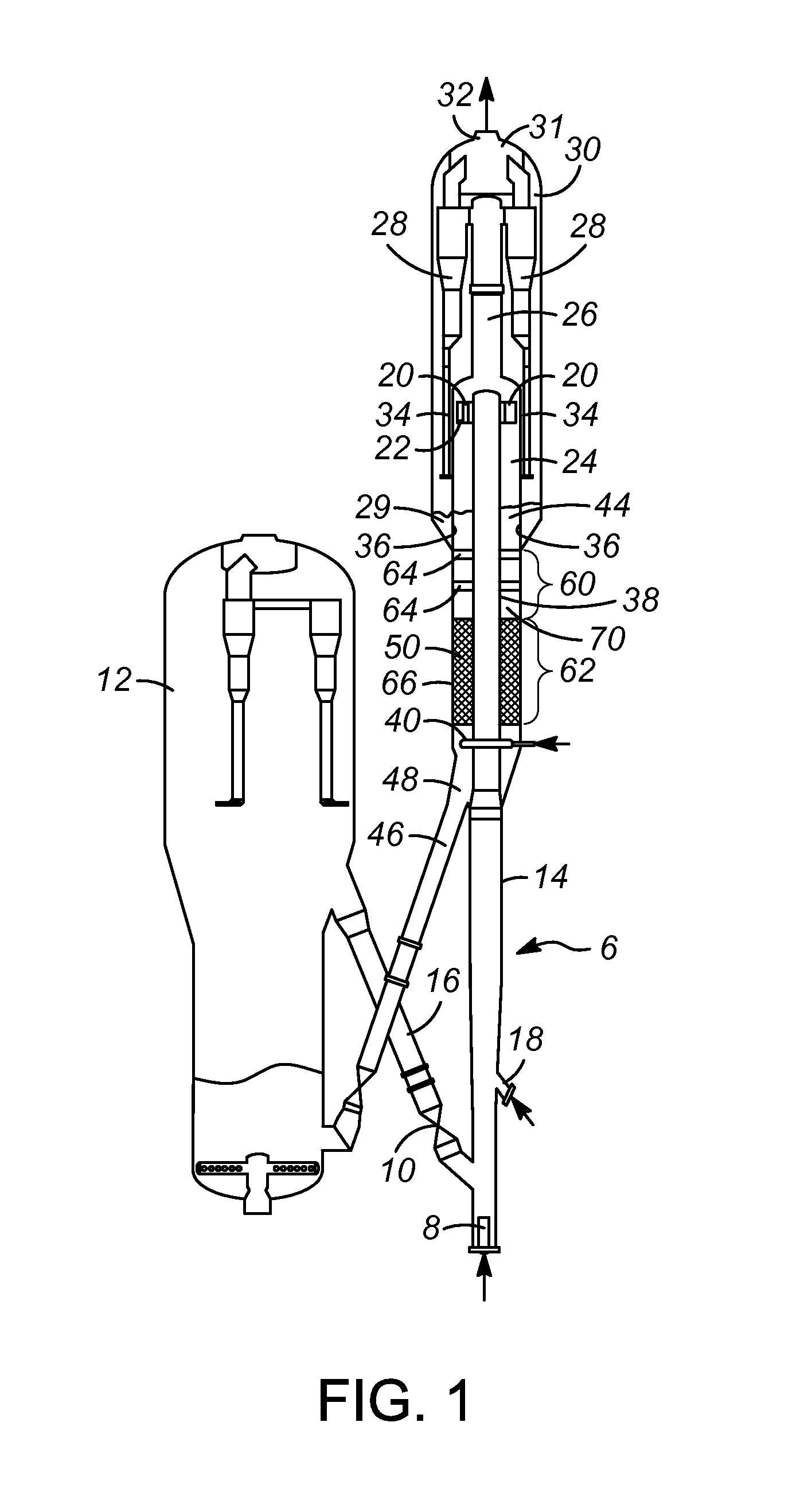
InactiveUS20050049604A1Minimize compressionMore processedJoint implantsSpinal implantsDistractionEndoscopic Procedure
A method and device for treating human intervertebral disc herniations using an endoscopic procedure. An access port is opened into and through the annulus of a disc to remove nucleus pulposus. Subsequent treatment, a balloon device having a valve structure is positioned via an endoscopic procedure into the disc space. The balloon device is filled with a physiological fluid to occupy the disc interspace or to maintain some degree of distraction of the created disc space. Post surgery, after fibrocollagenous tissue has grown into the disc space, a second endoscopic procedure is performed to remove the balloon device. Fluid is removed to collapse the balloon structure and then removed via the guide tube. The ingrowth of fibrocollagenous tissue will continue to fill the void formerly occupied by the balloon device. The balloon device has a rigid valve body and a flexible balloon member. The valve body has an end plug, a valve chamber holding a valve member and a cooperating biasing structure. The balloon member of the device is inserted into a vacated nucleus space of a herniated disc and then inflated with a physiological fluid using a fill tube. The balloon member, valve body, and fill tube or portions thereof may be constructed of a radiolucent material to provide visibility during implantation, surveillance and removal. The balloon device may be used in the cervical, lumbar or thoracic region of the spine.
Owner:PMT
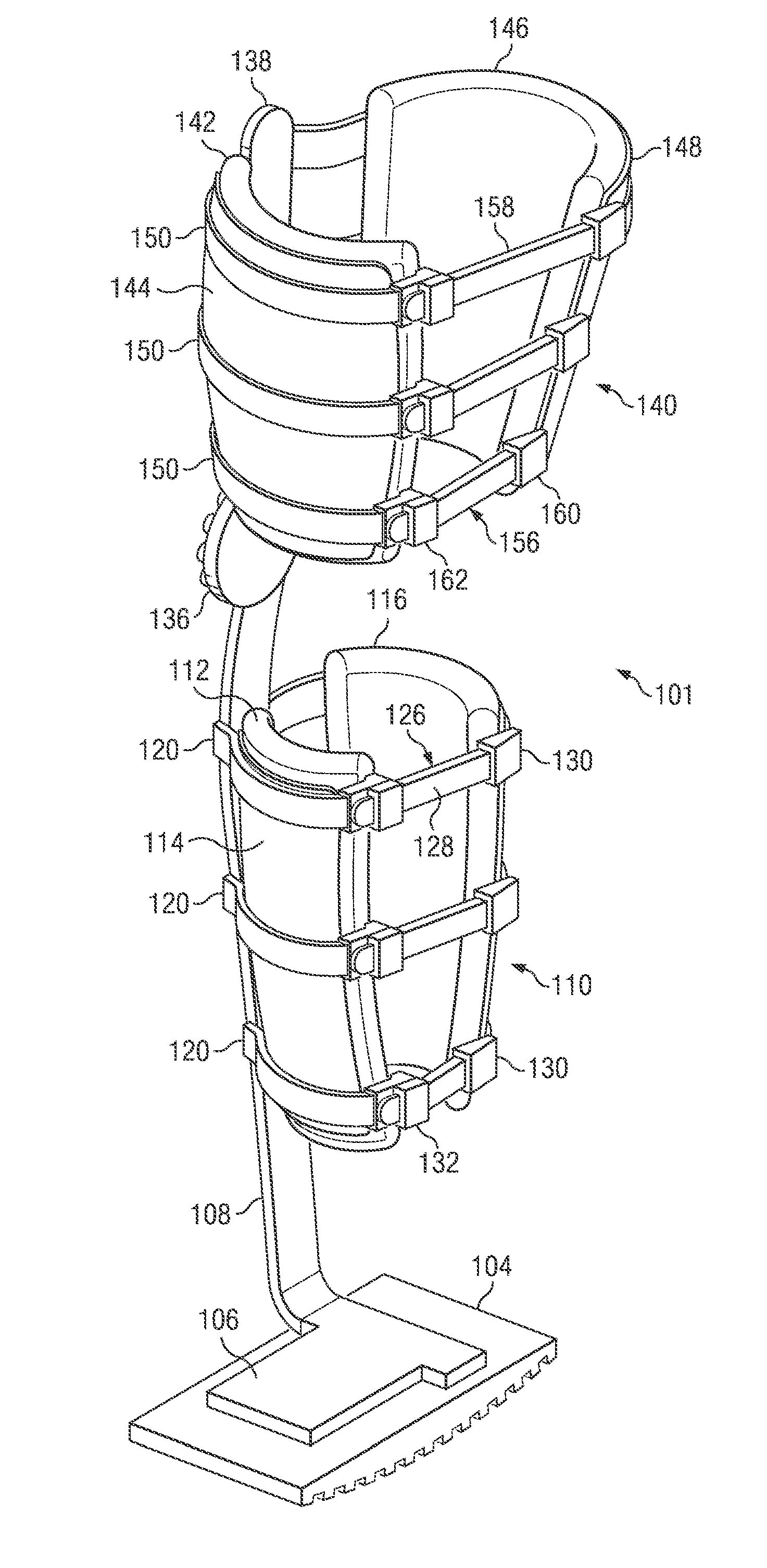
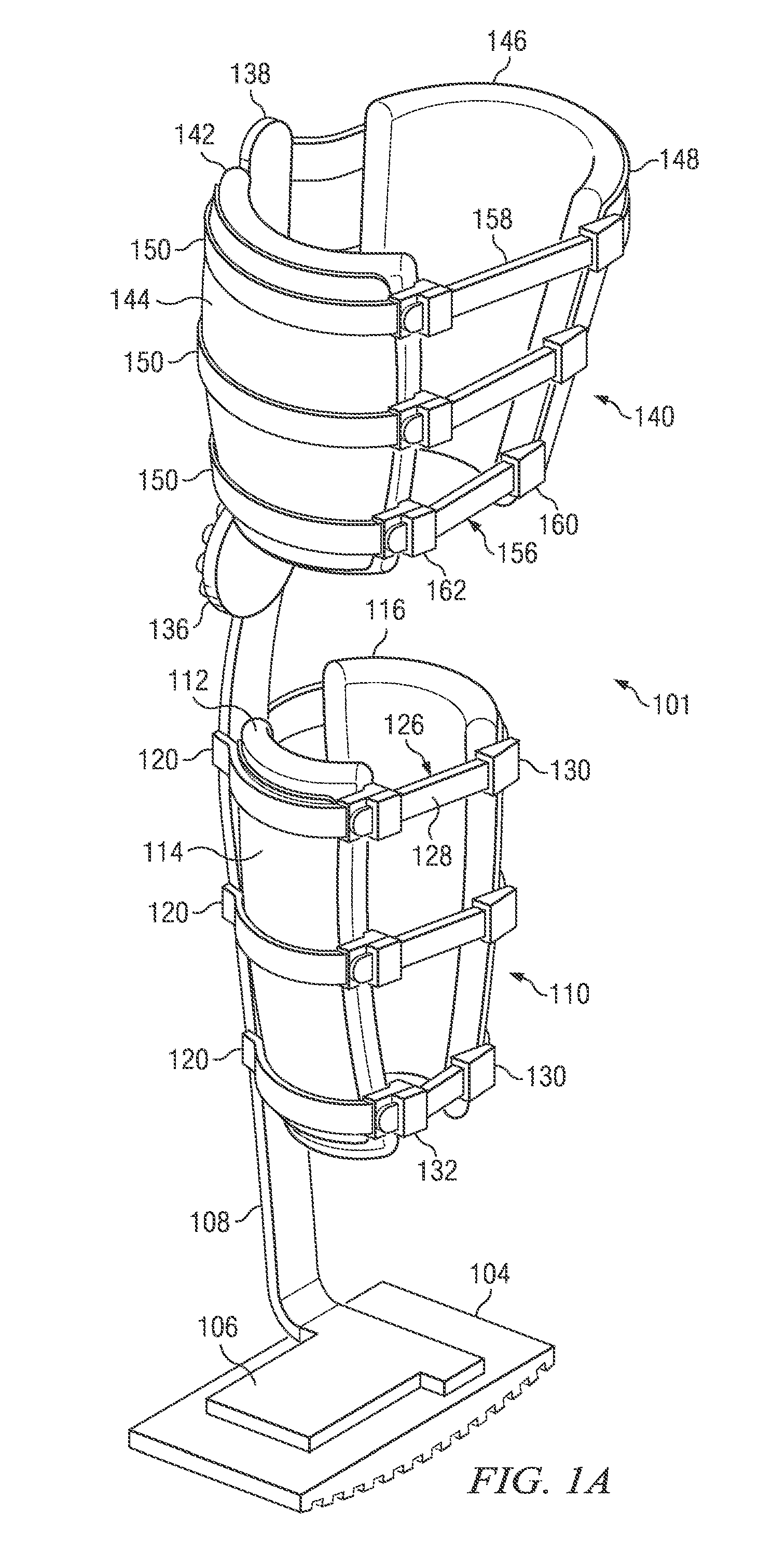
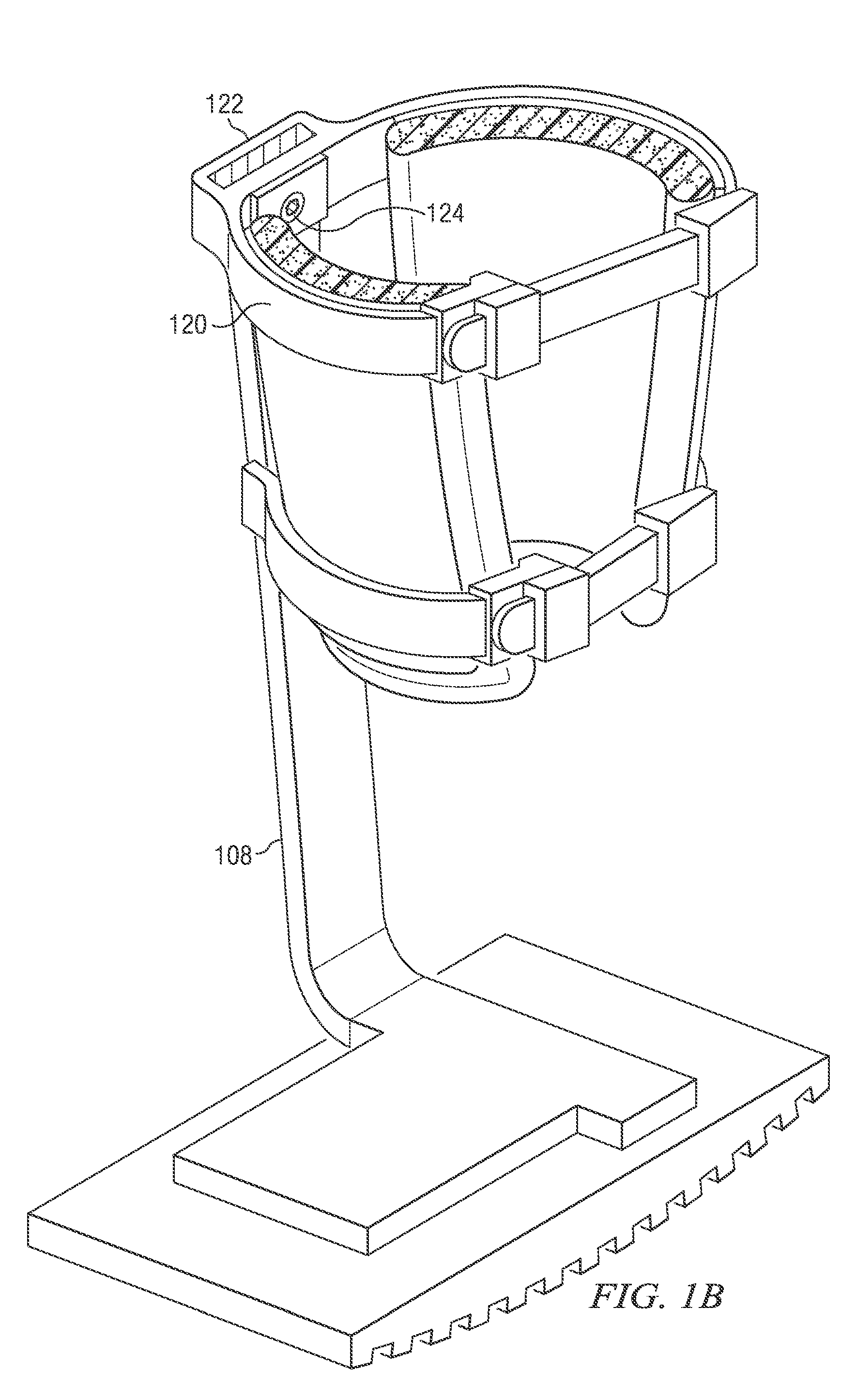
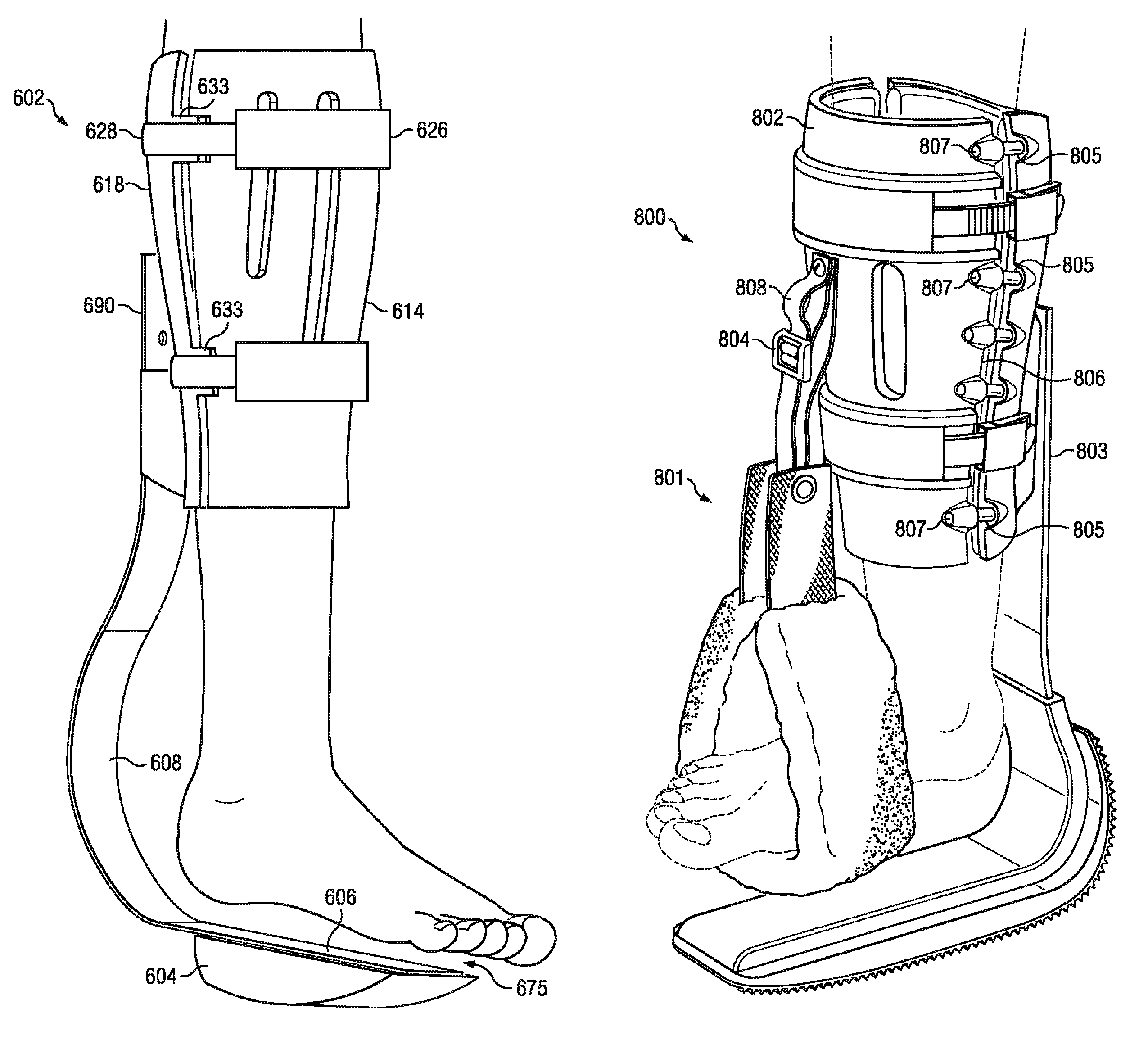
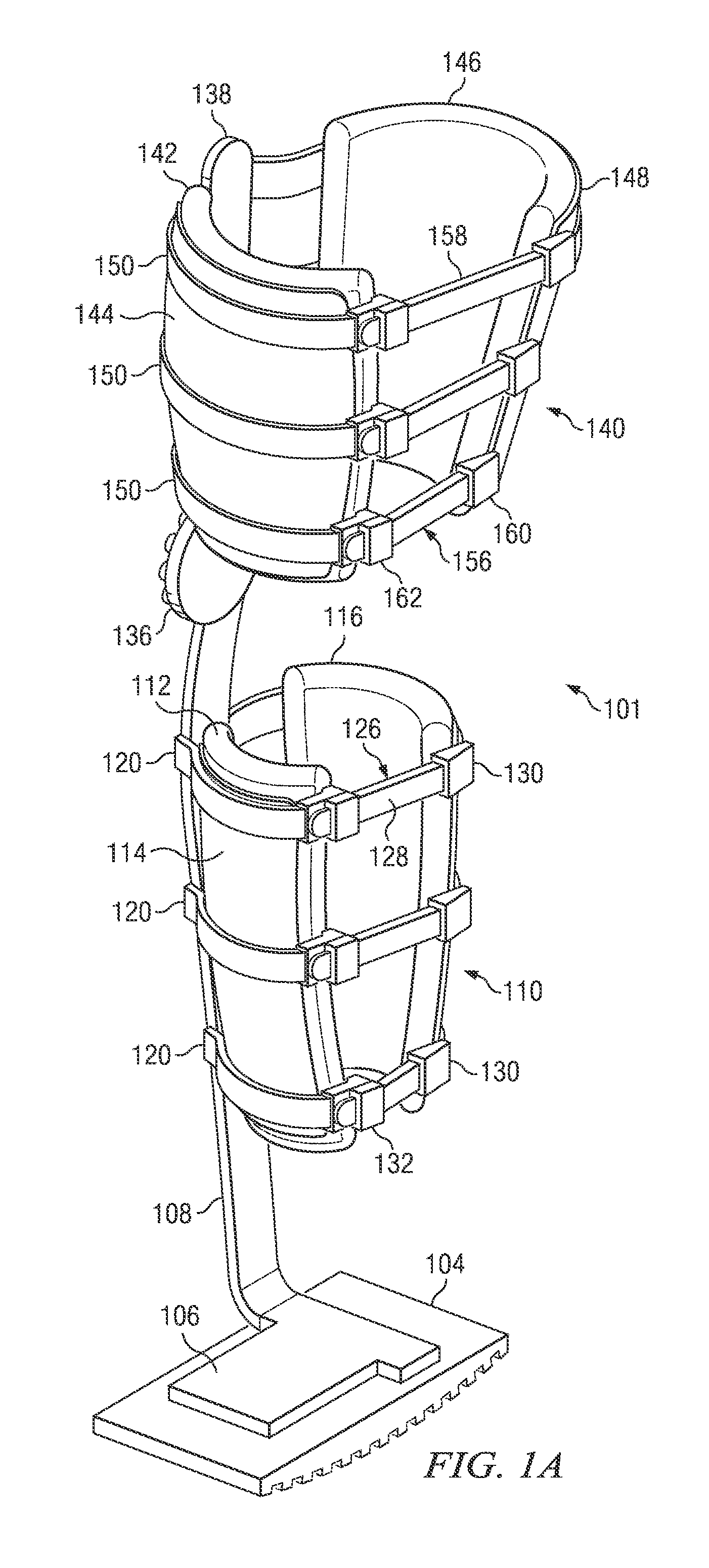
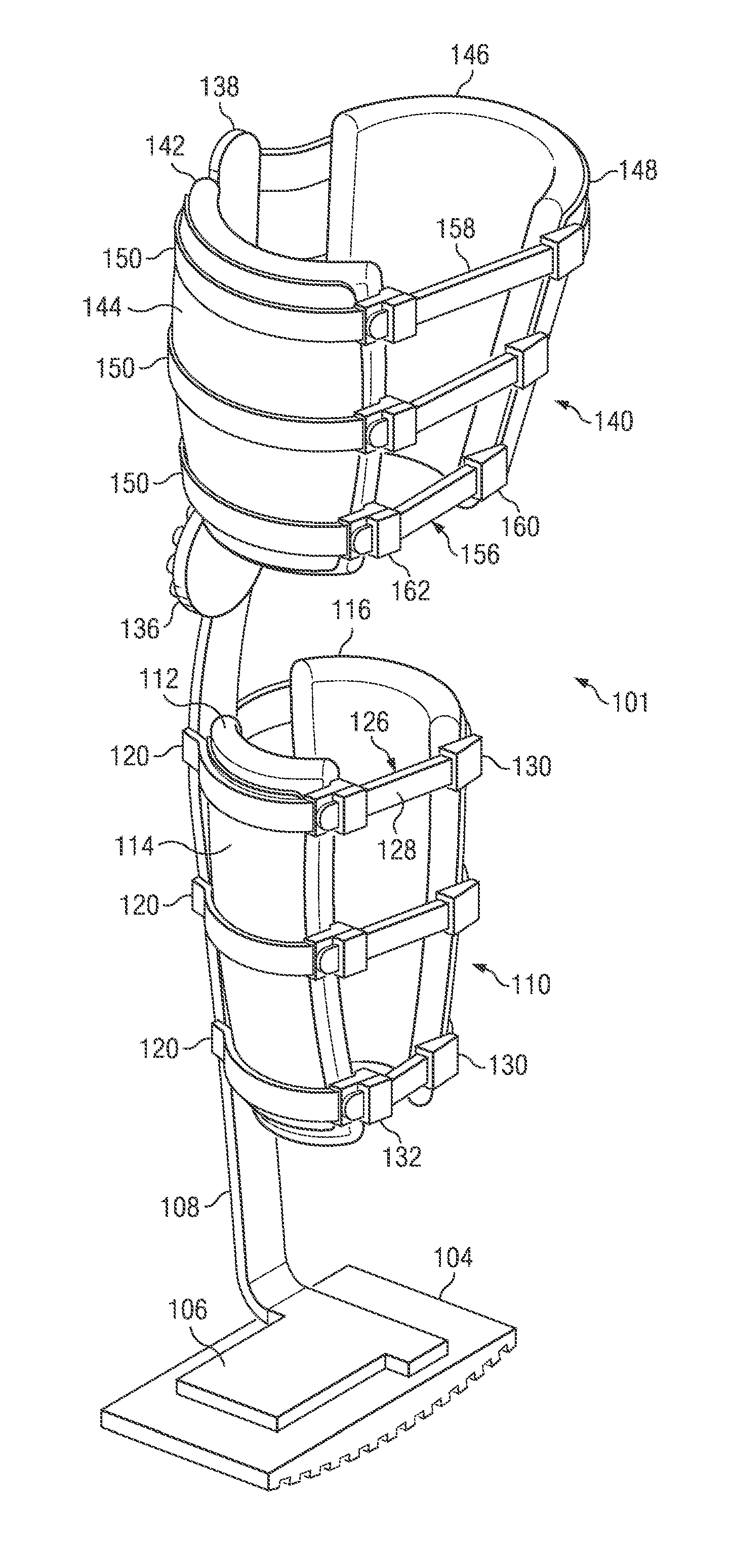
Weight-bearing lower extremity brace
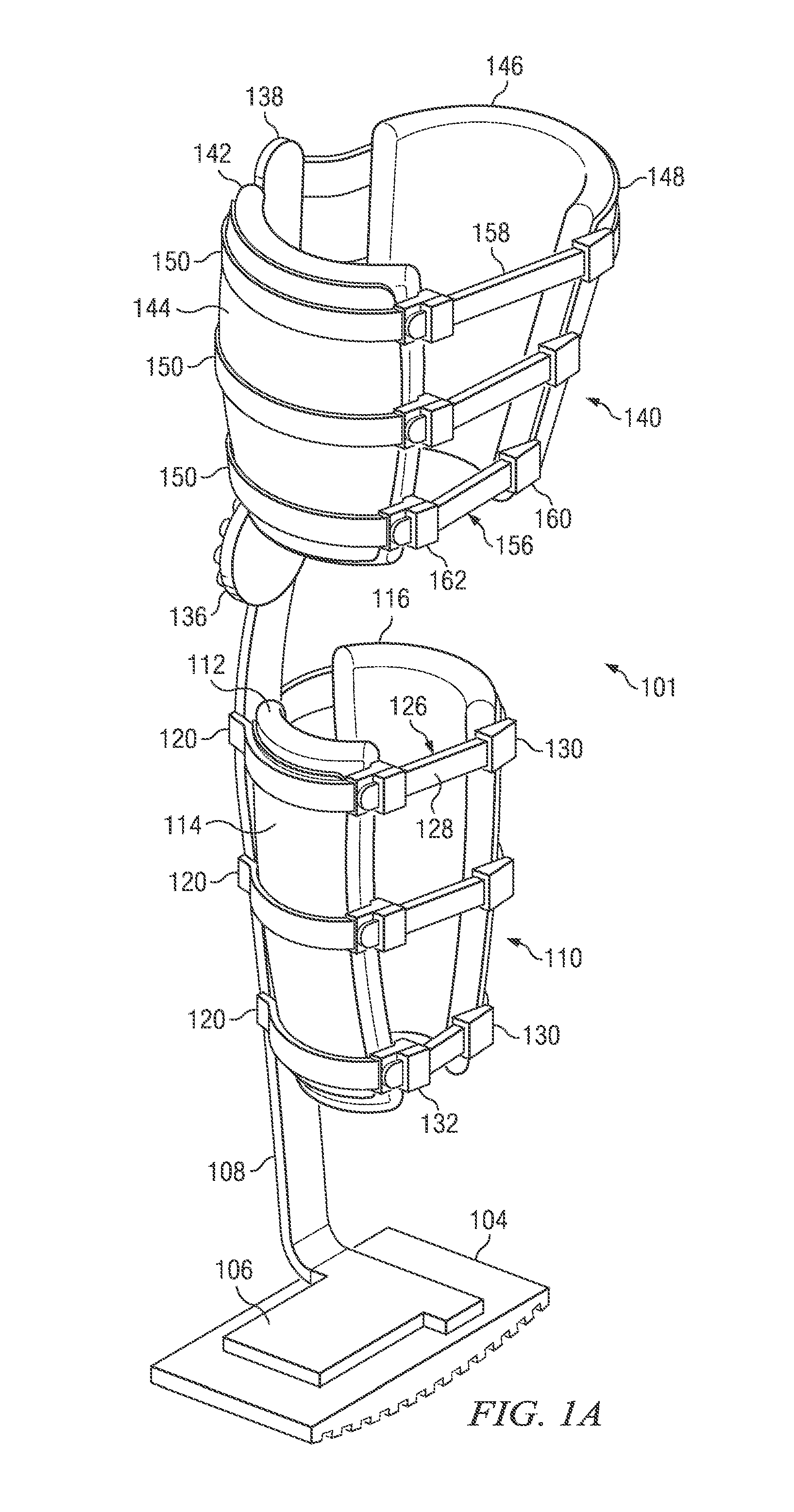
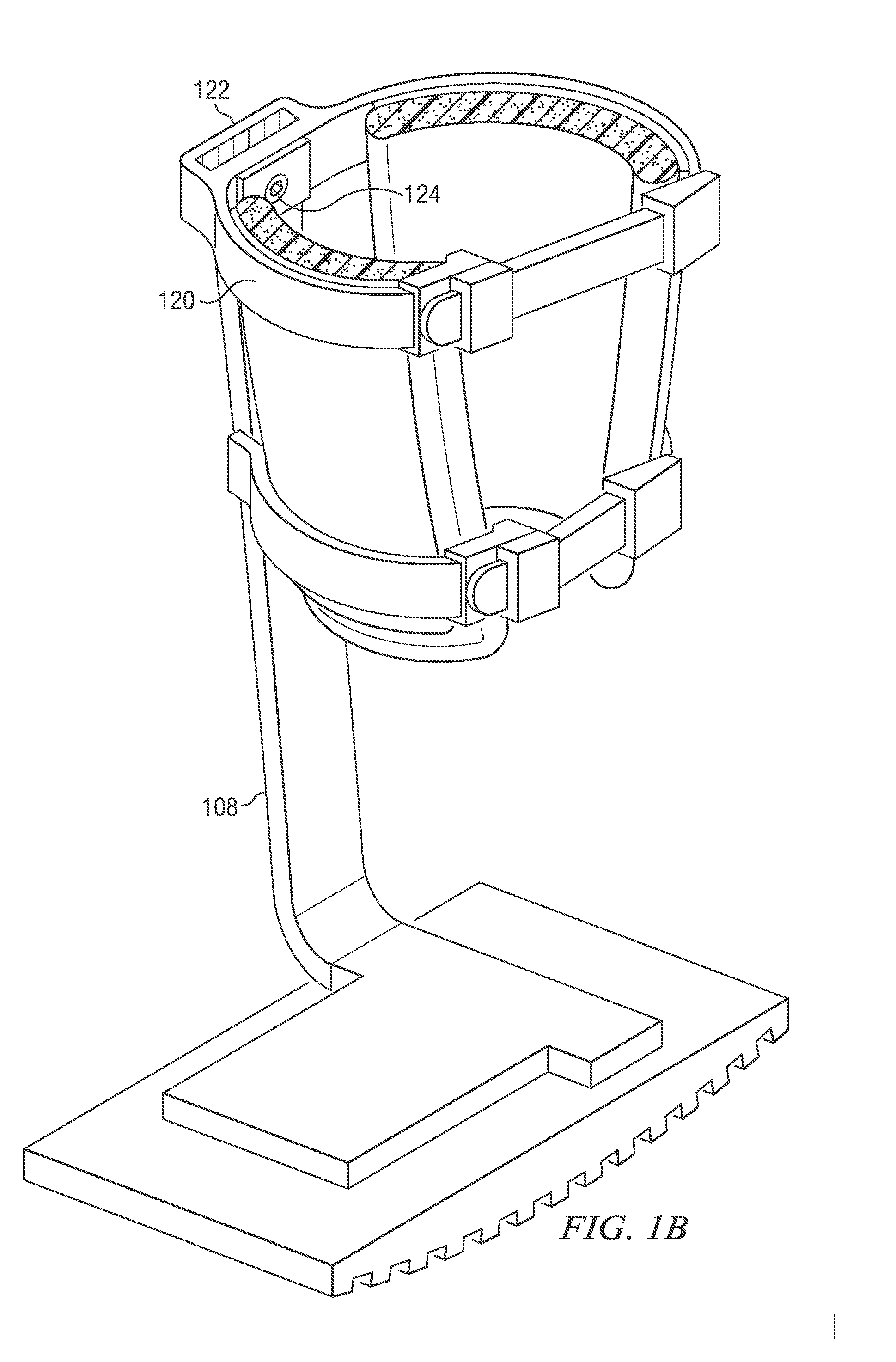
InactiveUS20130267878A1Minimize compressionReduce and eliminate effectNon-surgical orthopedic devicesArtificial legsEngineeringCuff
The present disclosure relates, according to some embodiments, to a device and / or system (e.g., redistributing weight away from a subject's foot), which may comprise (a) a platform, (b) at least one vertical support fixed to the platform and extending upwardly from the platform, and (c) at least one cuff (i) configured to surround and releasably grip at least a portion of a subject's leg other than the foot and (ii) mounted (e.g., adjustably) to the at least one vertical support at a vertical position along the at least one vertical support sufficient to suspend a subject's foot in a non-weight-bearing position above the platform during ambulation, wherein the platform, the at least one vertical support, and the at least one cuff together are configured to bear at least the subject's full weight.
Owner:TOAD MEDICAL
Weight-bearing lower extremity brace
InactiveUS8672865B2Minimize compressionReduce and eliminate effectPerson identificationNon-surgical orthopedic devicesEngineeringCuff
The present disclosure relates, according to some embodiments, to a device and / or system (e.g., redistributing weight away from a subject's foot), which may comprise (a) a platform, (b) at least one vertical support fixed to the platform and extending upwardly from the platform, and (c) at least one cuff (i) configured to surround and releasably grip at least a portion of a subject's leg other than the foot and (ii) mounted (e.g., adjustably) to the at least one vertical support at a vertical position along the at least one vertical support sufficient to suspend a subject's foot in a non-weight-bearing position above the platform during ambulation, wherein the platform, the at least one vertical support, and the at least one cuff together are configured to bear at least the subject's full weight.
Owner:TOAD MEDICAL
Device for treating intervertebral disc herniations
InactiveUS7128746B2Minimize compressionMore processedJoint implantsSpinal implantsDistractionEndoscopic Procedure
Owner:PMT
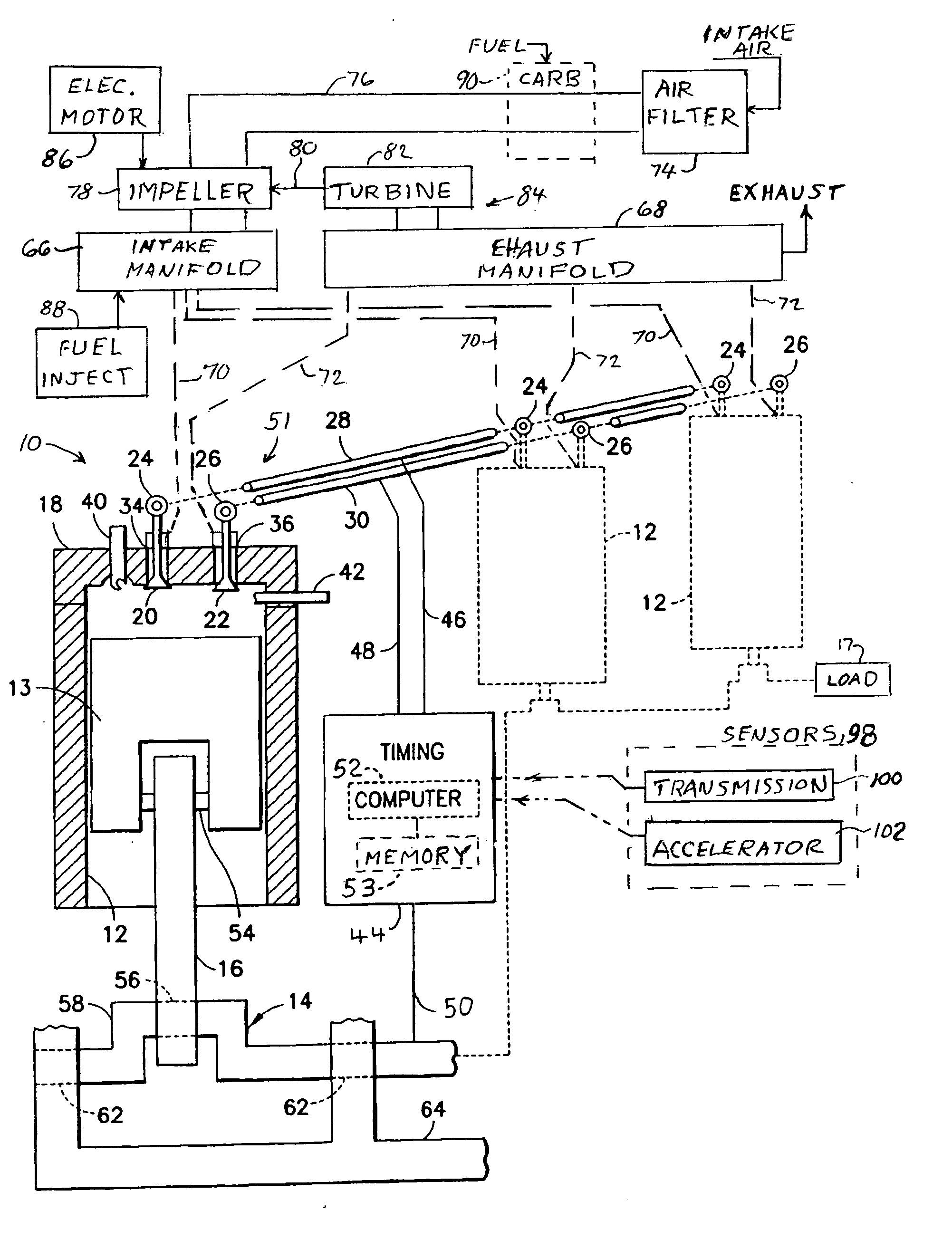
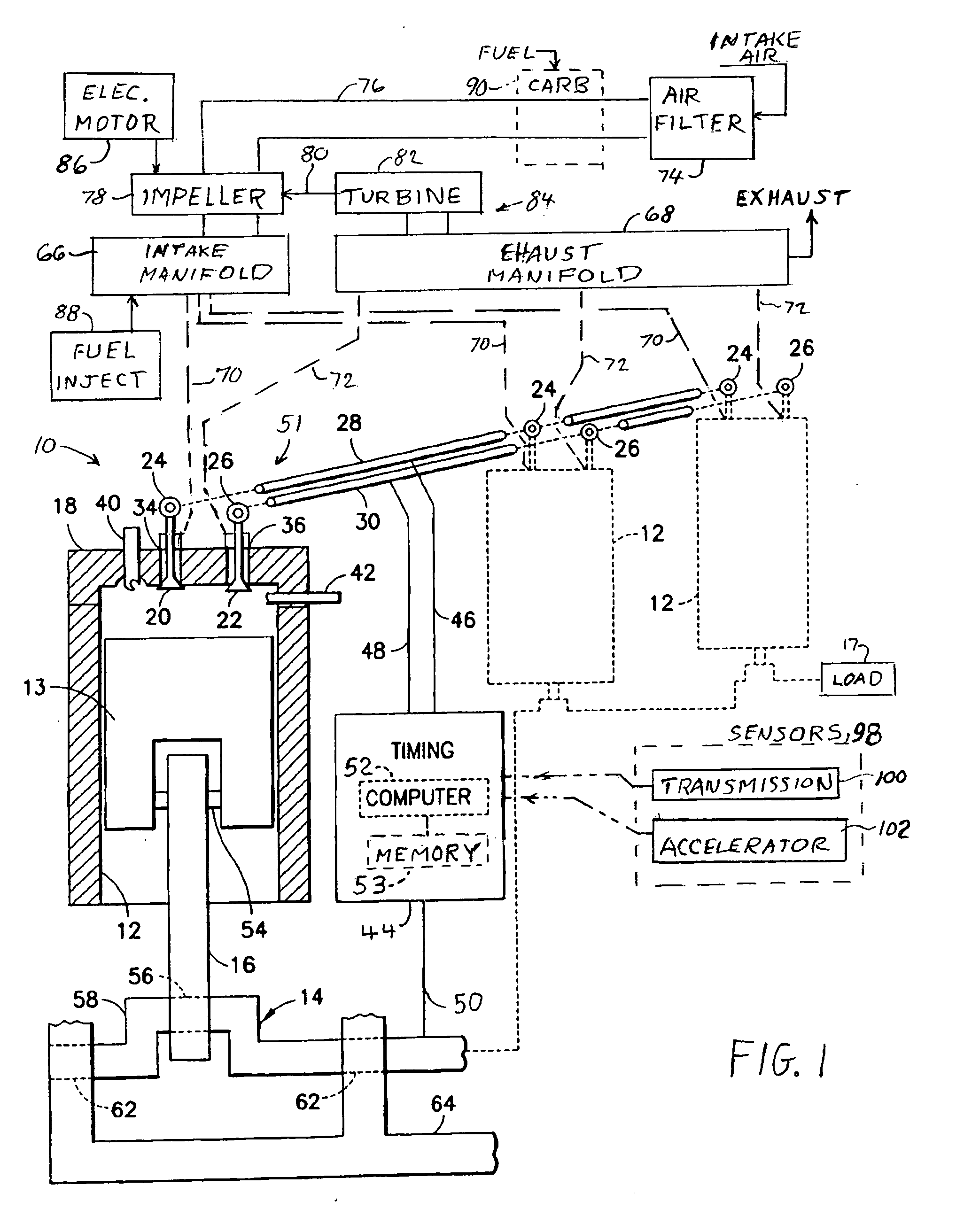
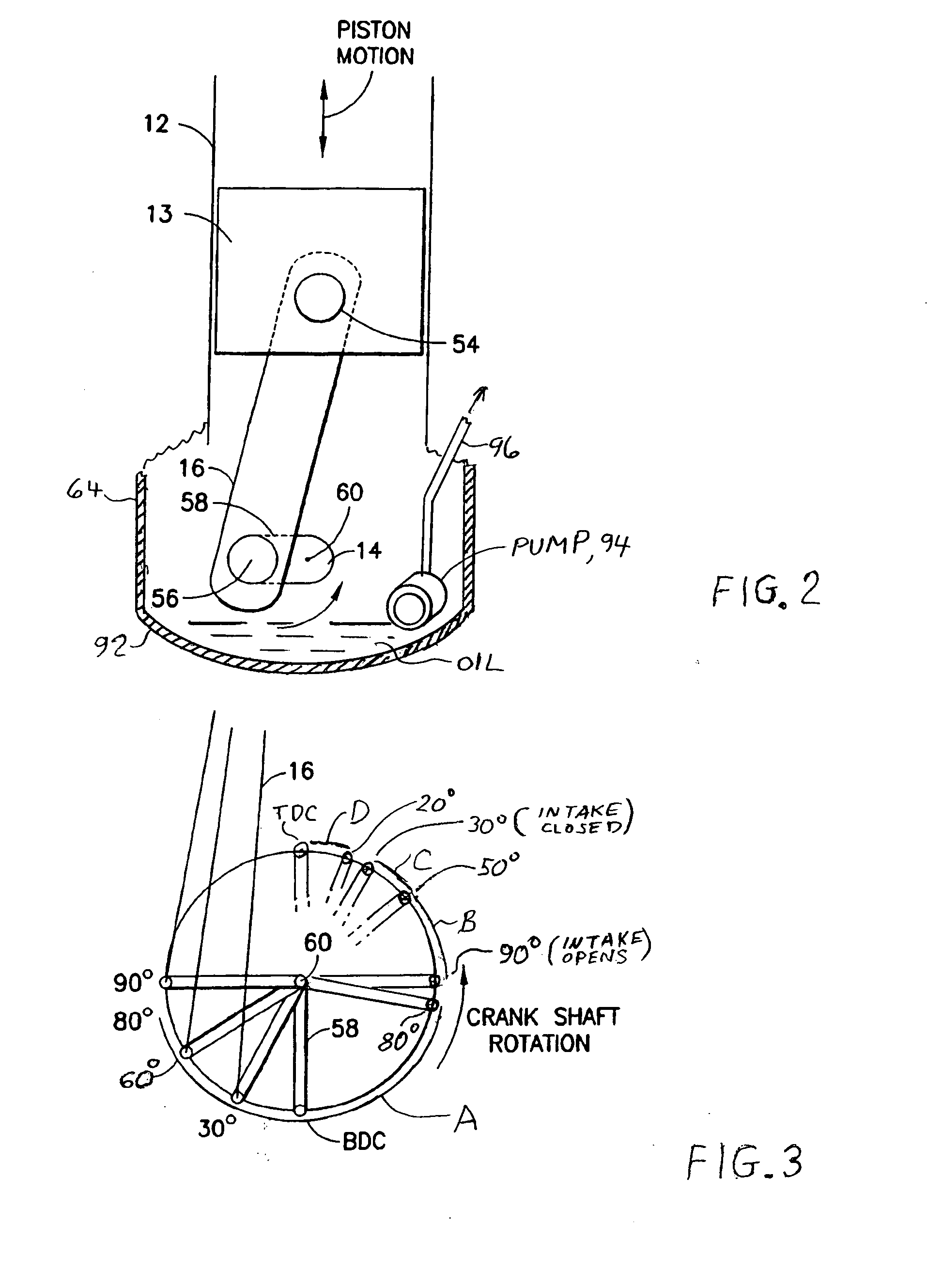
Two-stroke internal combustion engine with valves for improved fuel efficiency
InactiveUS20100037876A1Little movement of pistonMinimize compressionValve arrangementsInternal combustion piston enginesExhaust valveTop dead center
A two-stroke cycle internal combustion engine has a cylinder with a cylinder head closing off an end of the cylinder, a crankshaft and a piston connecting with the crankshaft to move the piston in a reciprocating movement within the cylinder. Intake and exhaust valves move into and out of a combustion chamber for openings and closures of the valves. A mixture of fuel and air is forced into the cylinder during an open interval of the intake valve beginning in the middle of the upstroke and terminating in a range of 20-60 degrees before top dead center to improve efficiency and choice of fuel by minimizing compressive forces of the piston during the upstroke. Timing of the closure of the intake valve may be delayed automatically with a reduction in crankshaft angular speed.
Owner:ROBINSON BARNETT JOEL
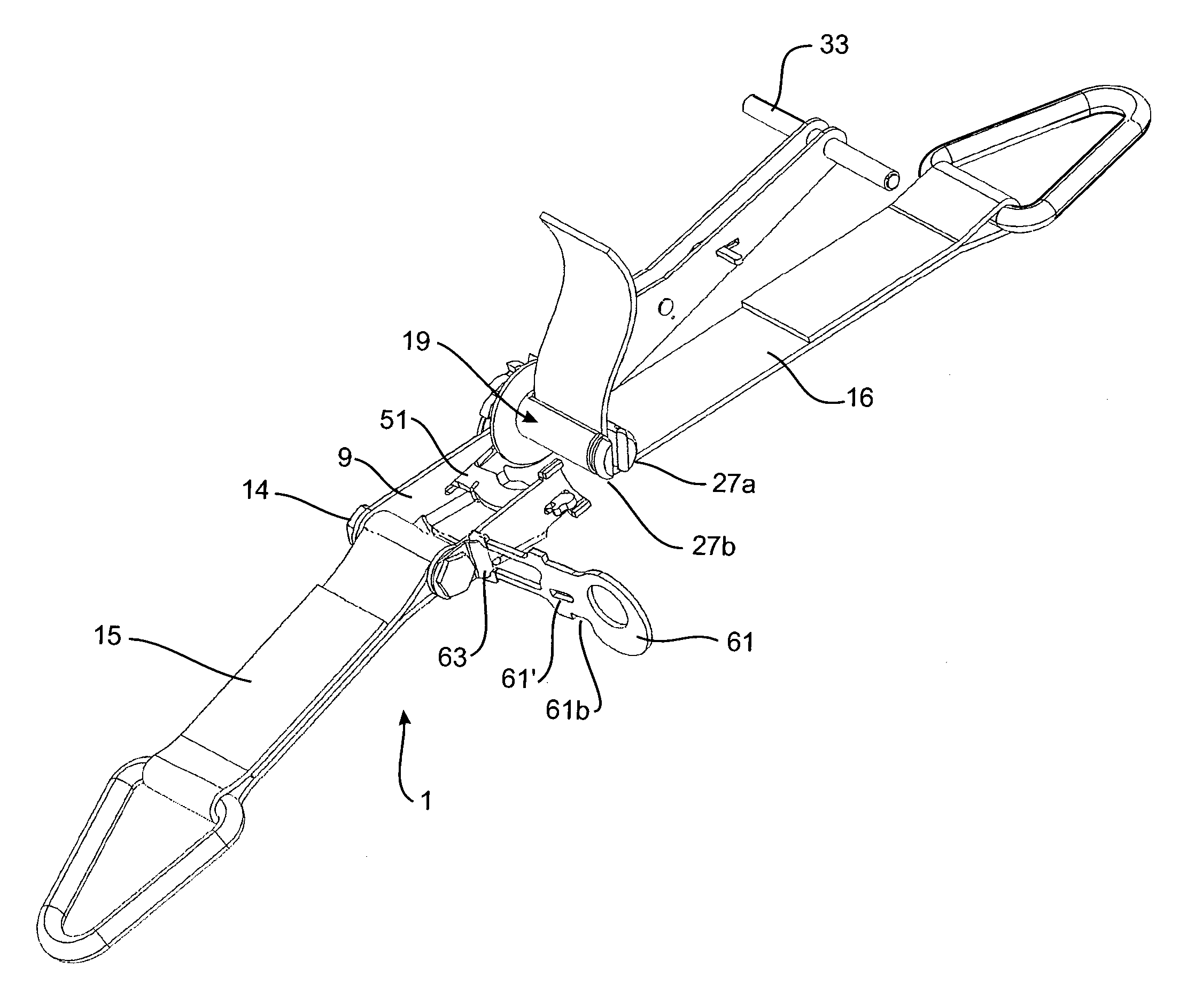
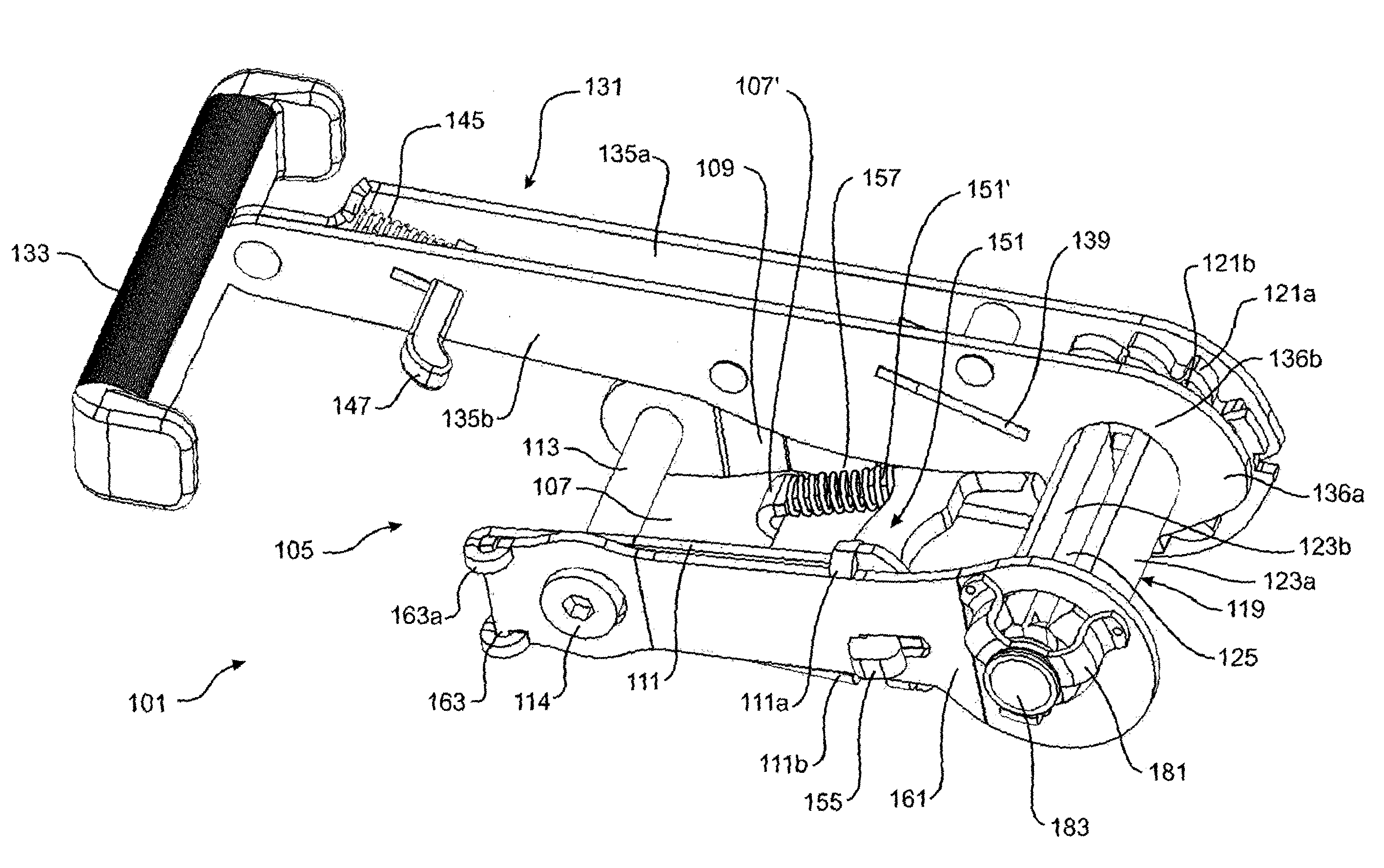
Side-loading ratchet device
ActiveUS9469239B2Avoid lateral loadMinimize compressionLifting devicesLoad securingEngineeringMechanical engineering
A ratchet device 1 for a strap has a body 105 having a side member 109, and a spool 119 having a side 119a rotatably supported by the side member and a ratchet wheel 121 fixed thereto. The body has a member 161 moveable between an open position in which the other side 119b of the spool is exposed to enable a strap to be laterally loaded into the spool 119 from the exposed side of the spool and laterally unloaded from the spool, and a closed position in which said lateral loading and unloading are prevented.
Owner:ARMOR HLDG
Stripping vessel for removing hydrocarbons entrained in catalyst particles
ActiveUS20160158741A1Negatively impacting the flow of catalyst andMinimizes accumulationMolecular sieve catalystsOther chemical processesMarine engineeringStructured packing
A stripping vessel for removing hydrocarbons from a catalyst and a process for removing hydrocarbons from a catalyst. In an FCC unit, the stripping vessel includes first and second stripping sections. The first stripping section includes at least one grid having a plurality of interesting members and openings therebetween. The second stripping section includes structured packing such as a plurality of ribbons. The one or more grids are spaced from the structured packing, and from each other, so as to minimize the accumulation of catalyst within the stripping vessel, preferably between about 0.91 m (3 ft) to about 1.5 m (5 ft).
Owner:UOP LLC
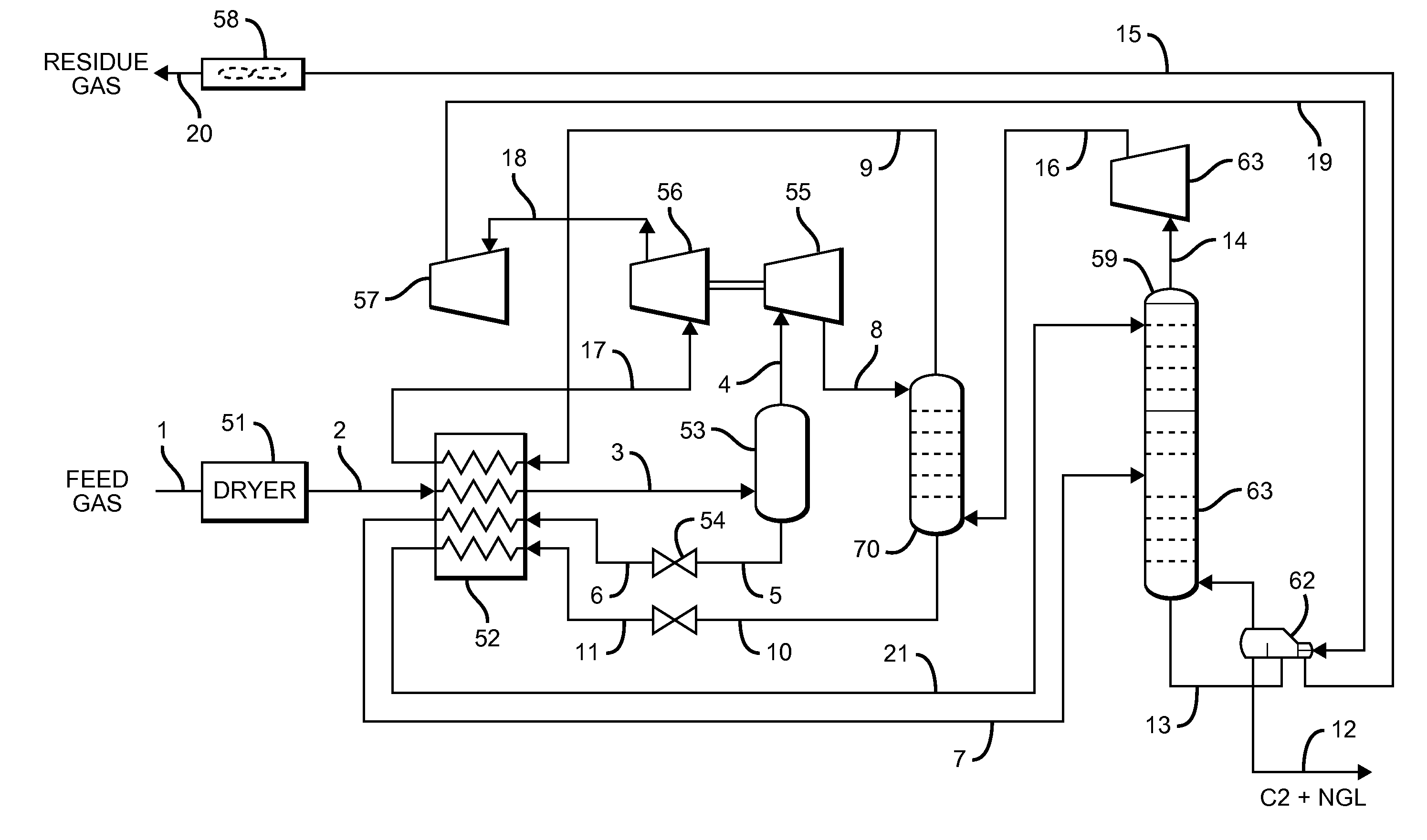
Configurations and methods for offshore ngl recovery
InactiveUS20140060114A1Minimizing overall compression horsepowerLess heating requirementSolidificationLiquefactionTurboexpanderHigh pressure
A natural gas two-column processing plant allows for recovery of at least 95% of C4 and heavier hydrocarbons, and about 60 to 80% of C3 hydrocarbons from a rich feed gas stream in which the first column (absorber) operates at a higher pressure than the second column, with the absorber receiving a compressed gas from the second column, and a turboexpander discharging a two-phase stream to the top of the absorber. Most typically, contemplated configurations and methods operate without the use of external refrigeration.
Owner:FLUOR TECH CORP
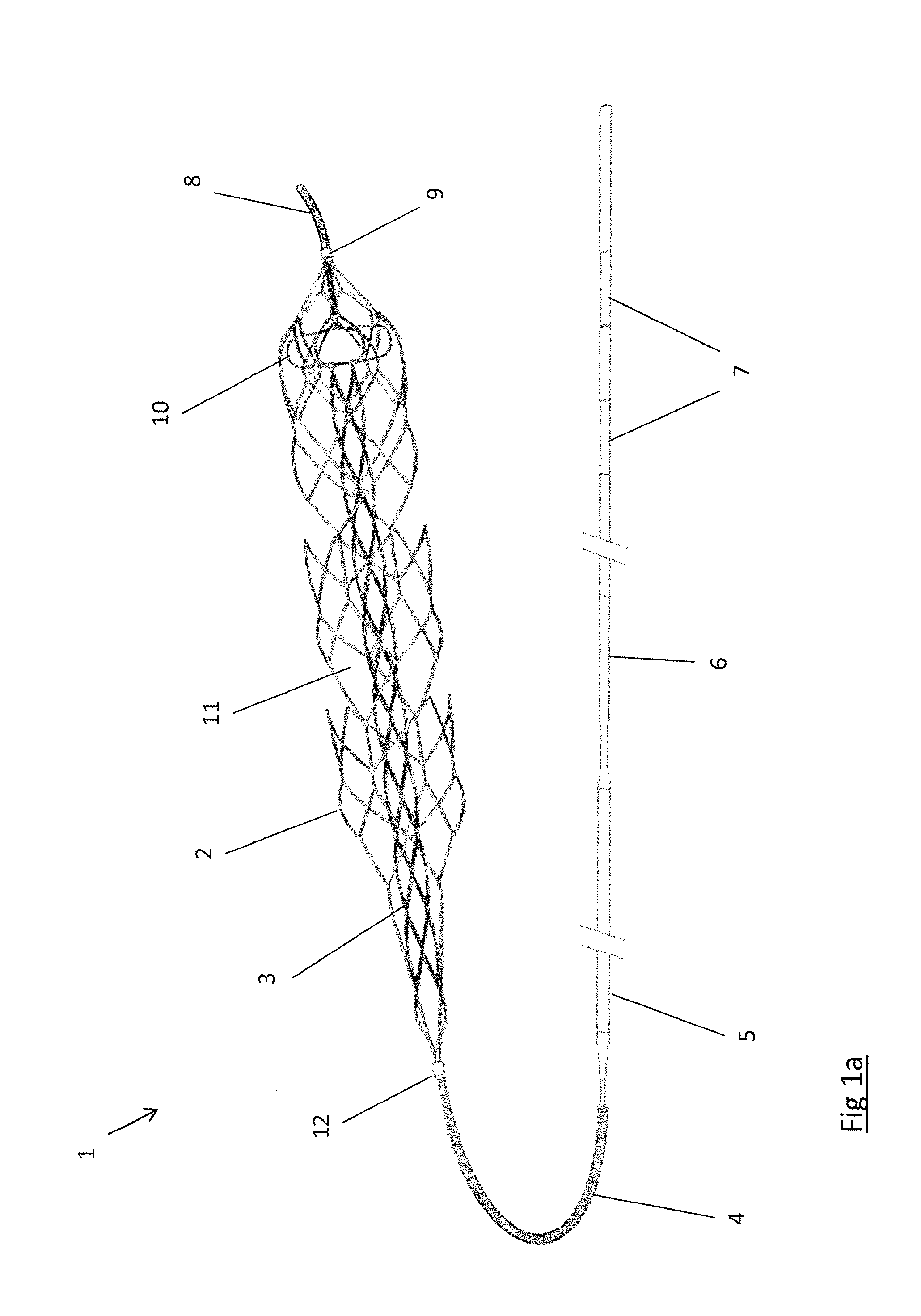
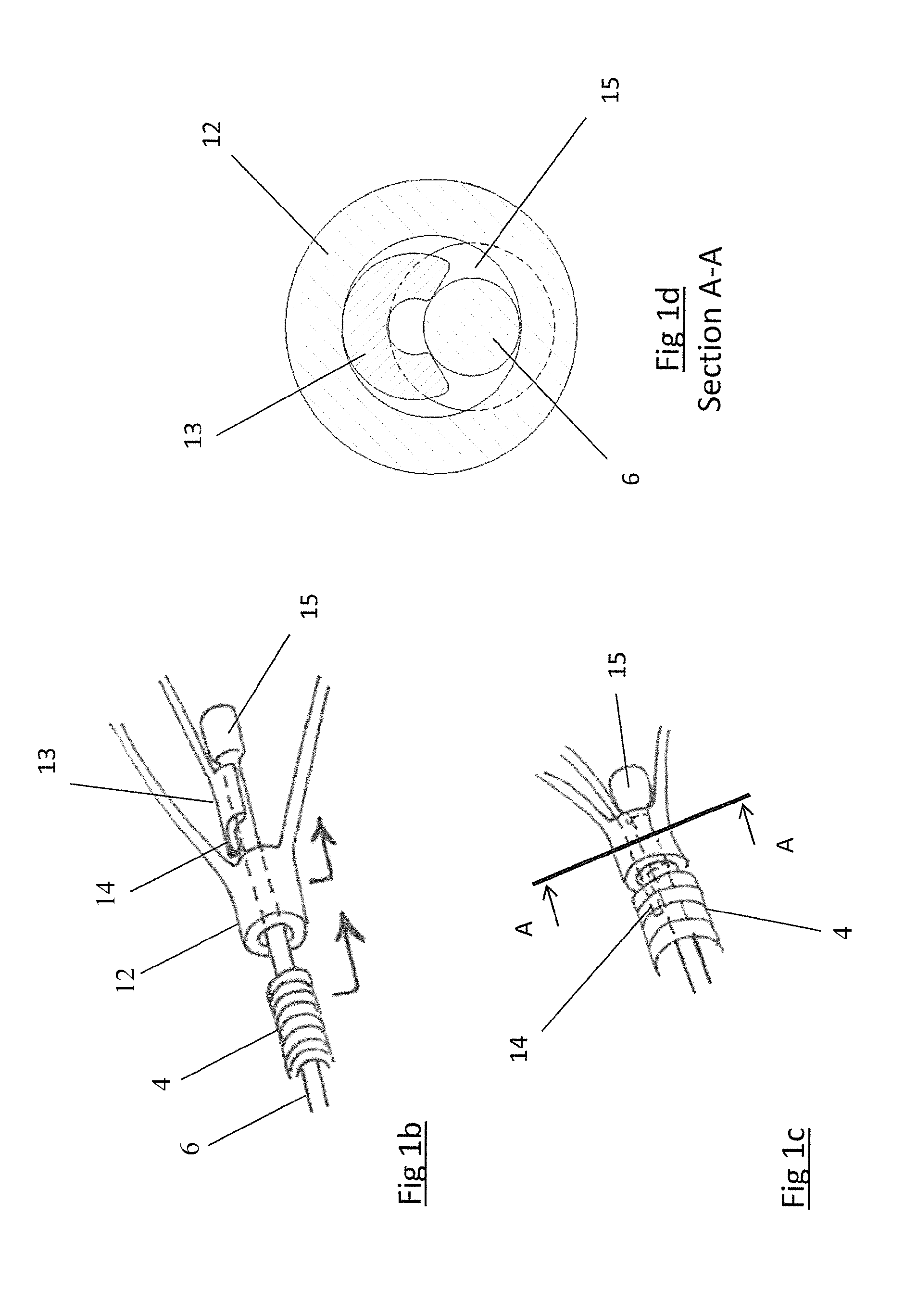
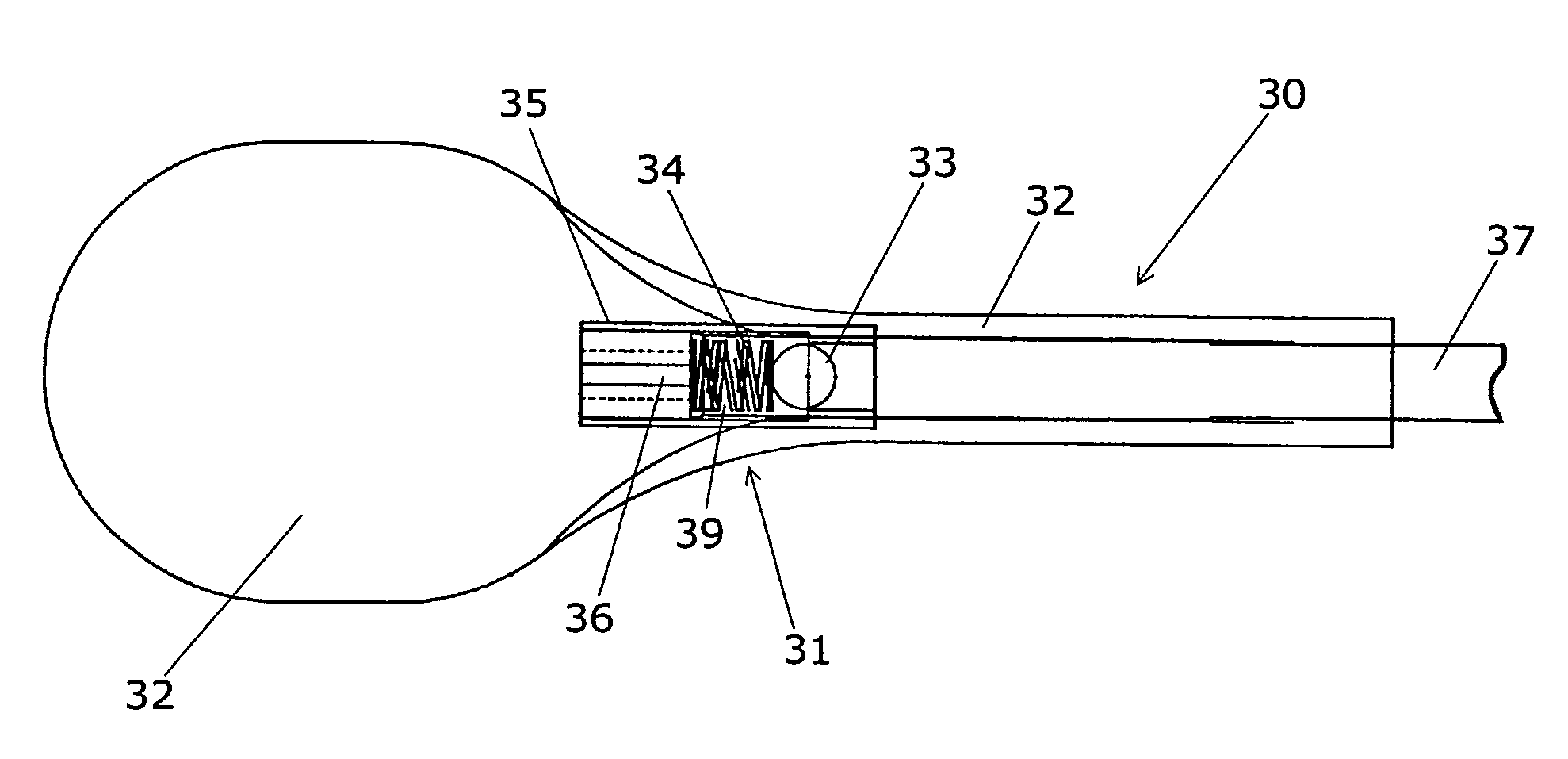
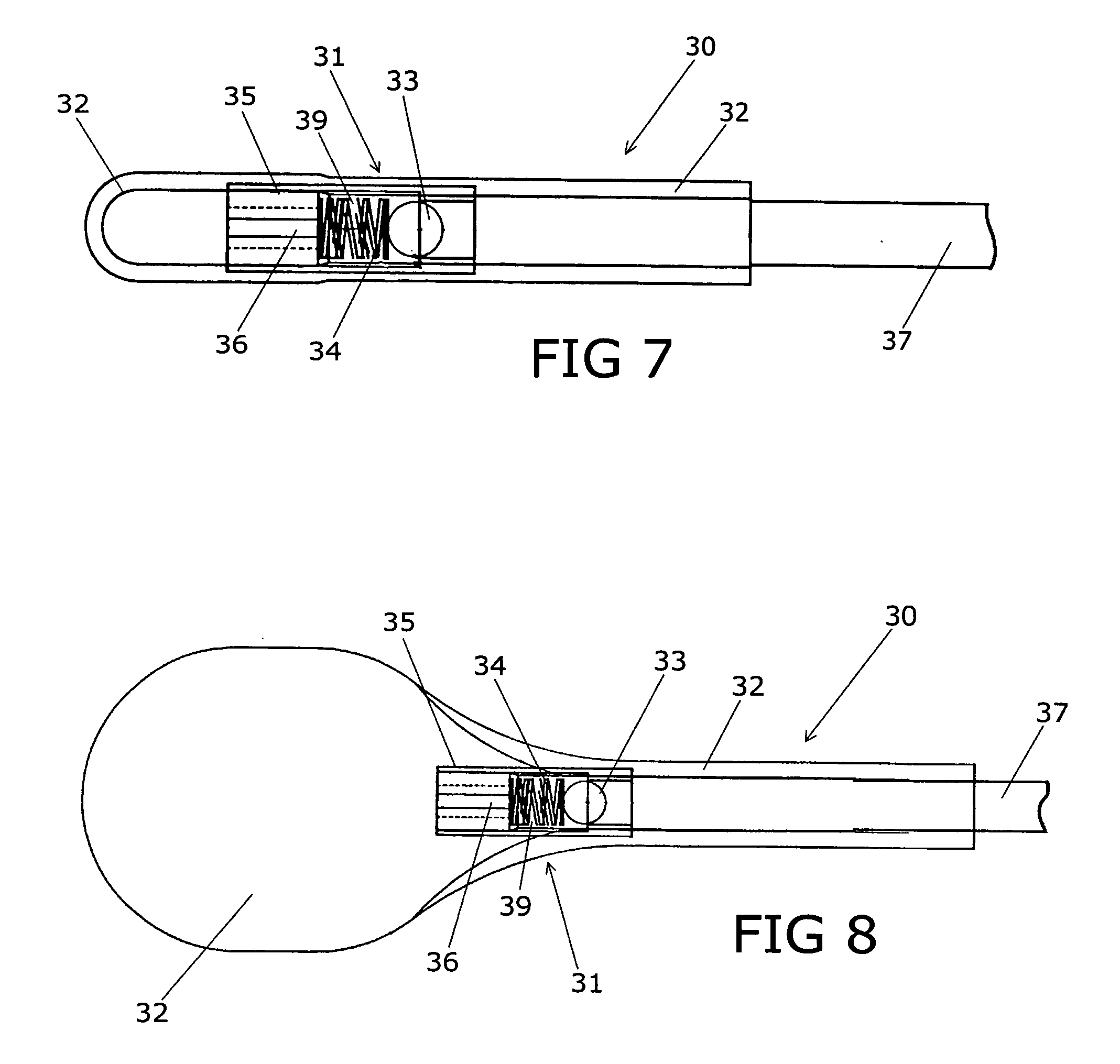
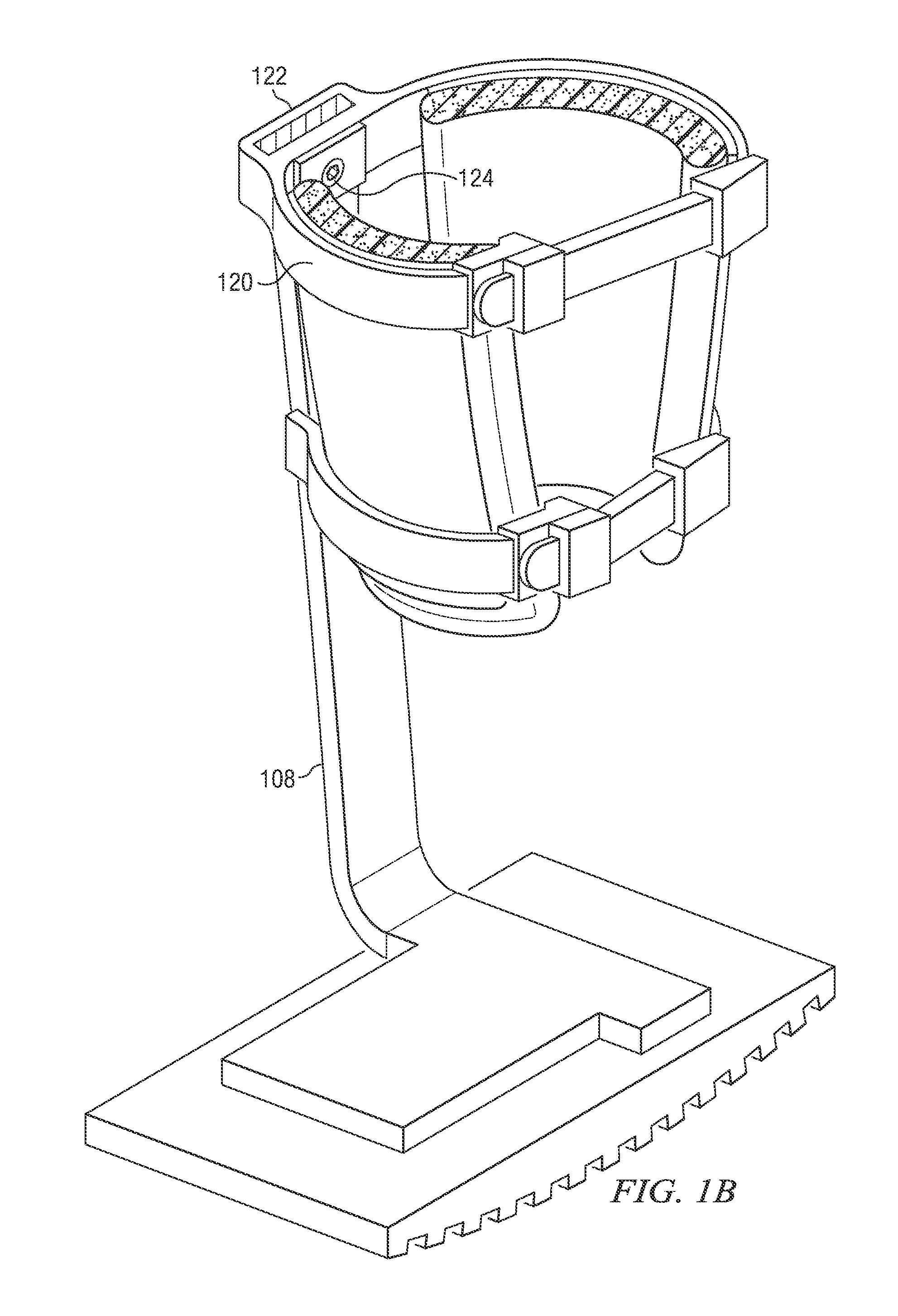
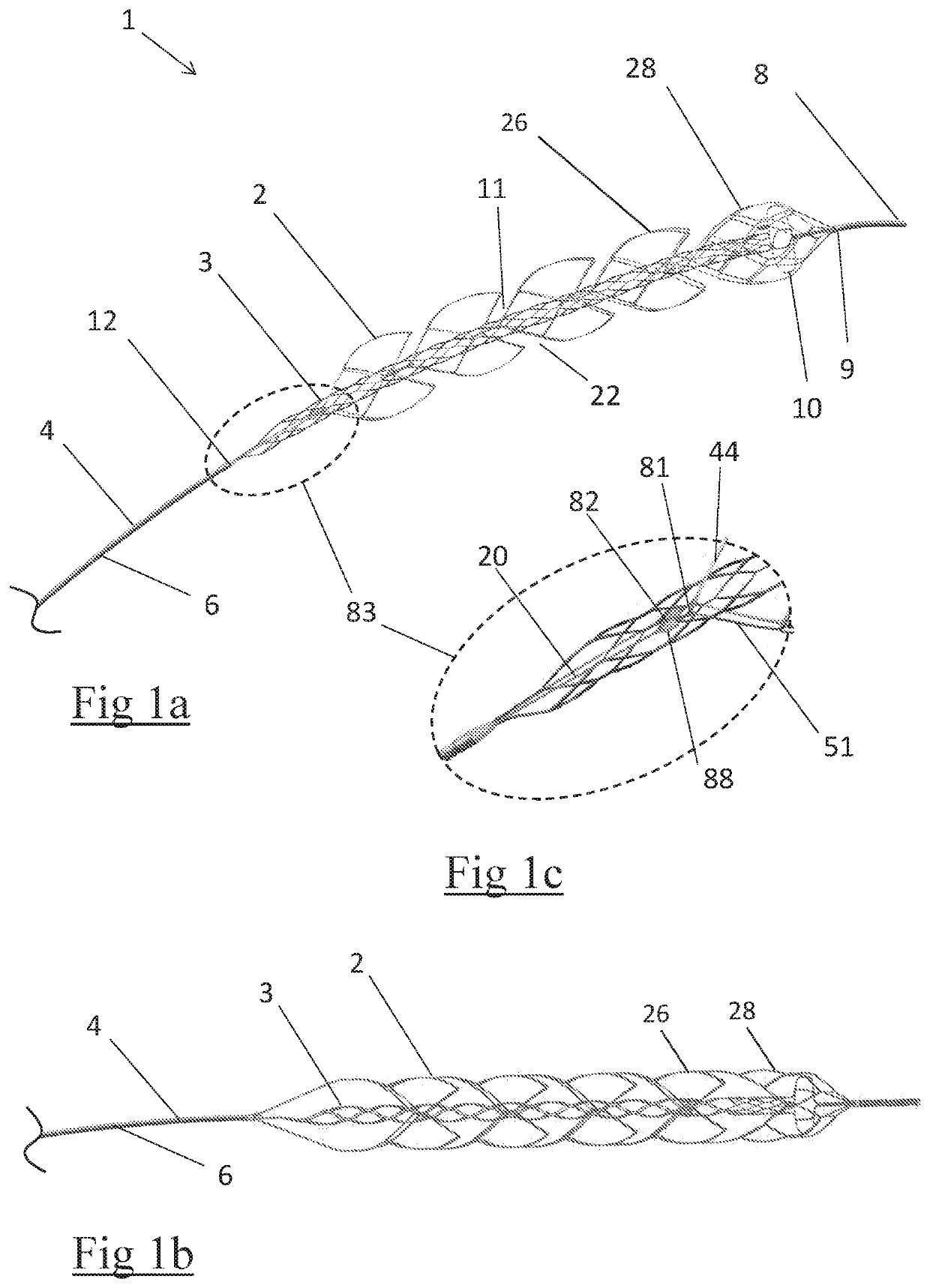
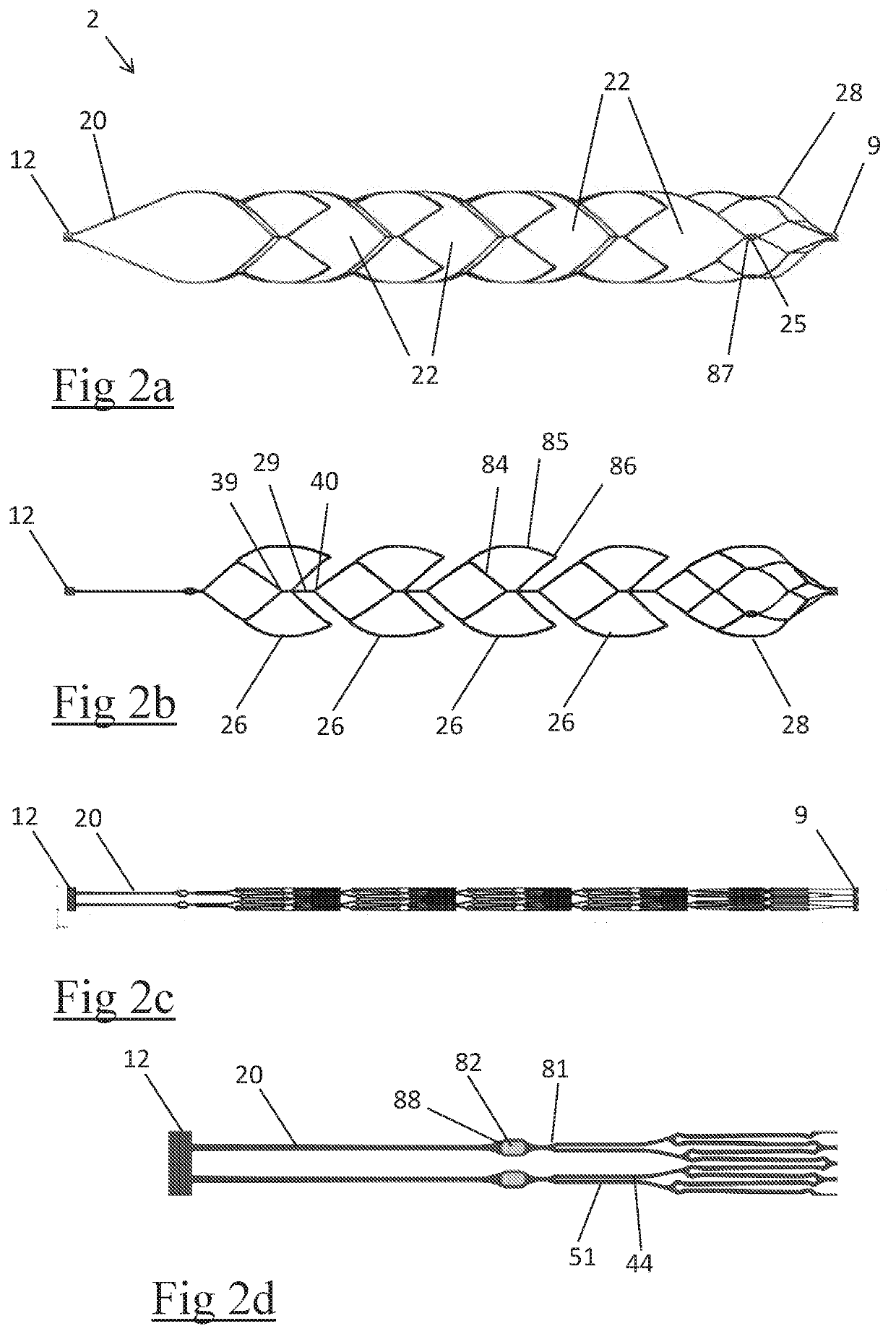
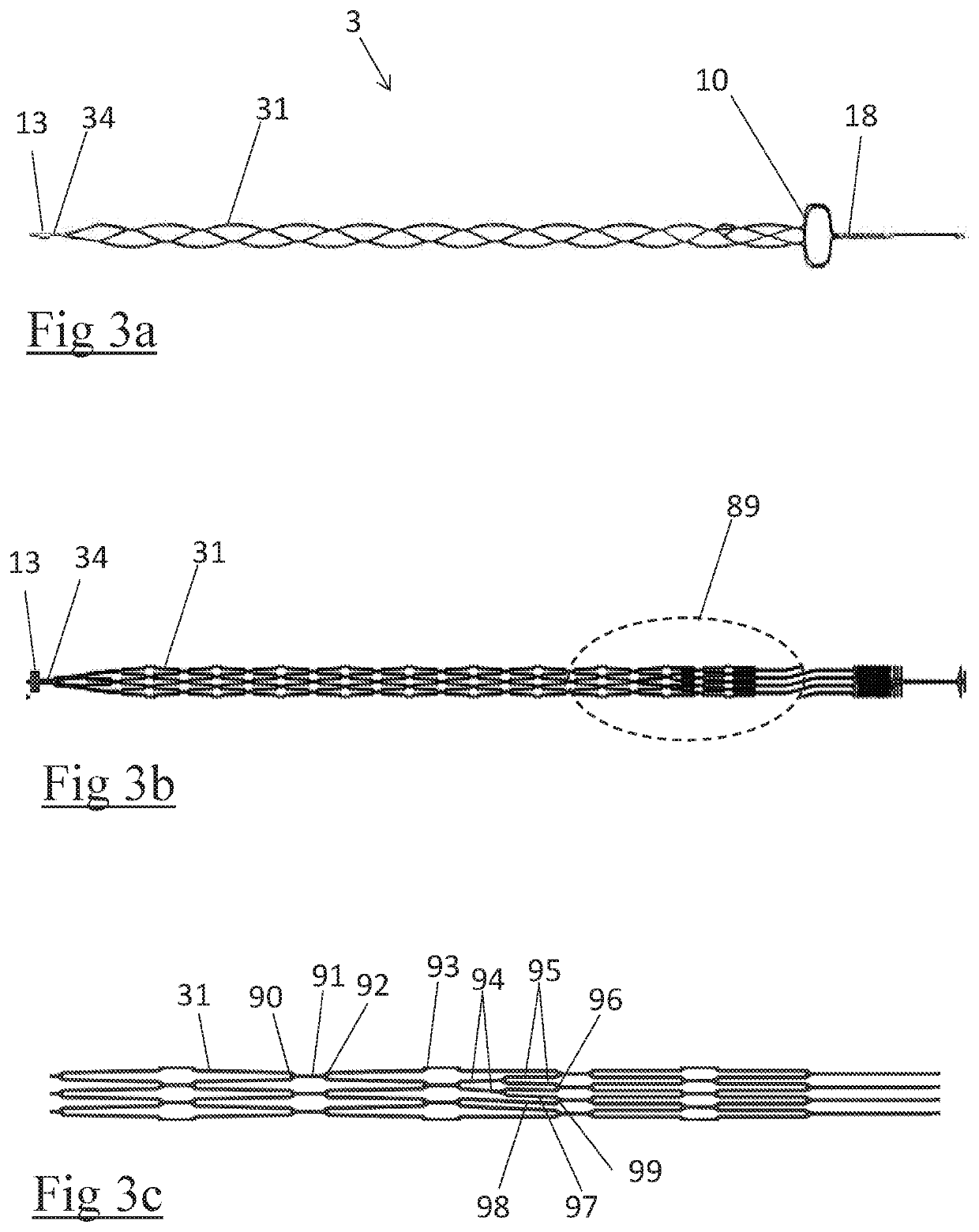
A clot retrieval device for removing occlusive clot from a blood vessel
ActiveUS20200305900A1Reduce gradientEasy to disassembleDiagnosticsExcision instrumentsSurgeryBiomedical engineering
A clot retrieval device for removing occlusive clot from a blood vessel. The device comprises an inner elongate body having a collapsed delivery configuration and an expanded deployed configuration. An outer elongate body is at least partially overlying the inner elongate body. The outer elongate body is expandable to a radial extent which is greater than the radial extent of the inner body in the deployed configuration to define a clot reception space. The outer elongate body has a plurality of clot receiving openings and a plurality of clot engaging regions. The clot engaging regions are adapted, on engagement with clot, to urge clot towards the clot receiving openings and into the reception space between the outer elongate body and the inner elongate body, wherein the radial force profile of the device varies along the length of the device.
Owner:NEURAVI
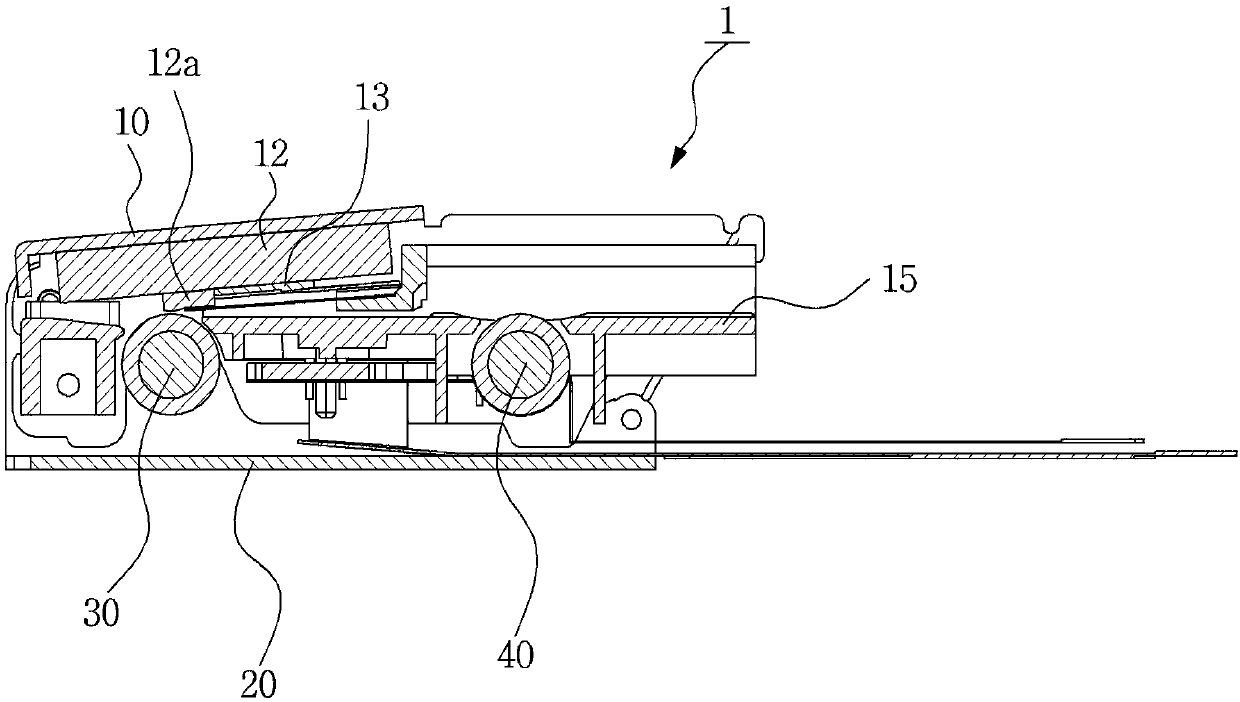
Photo printer
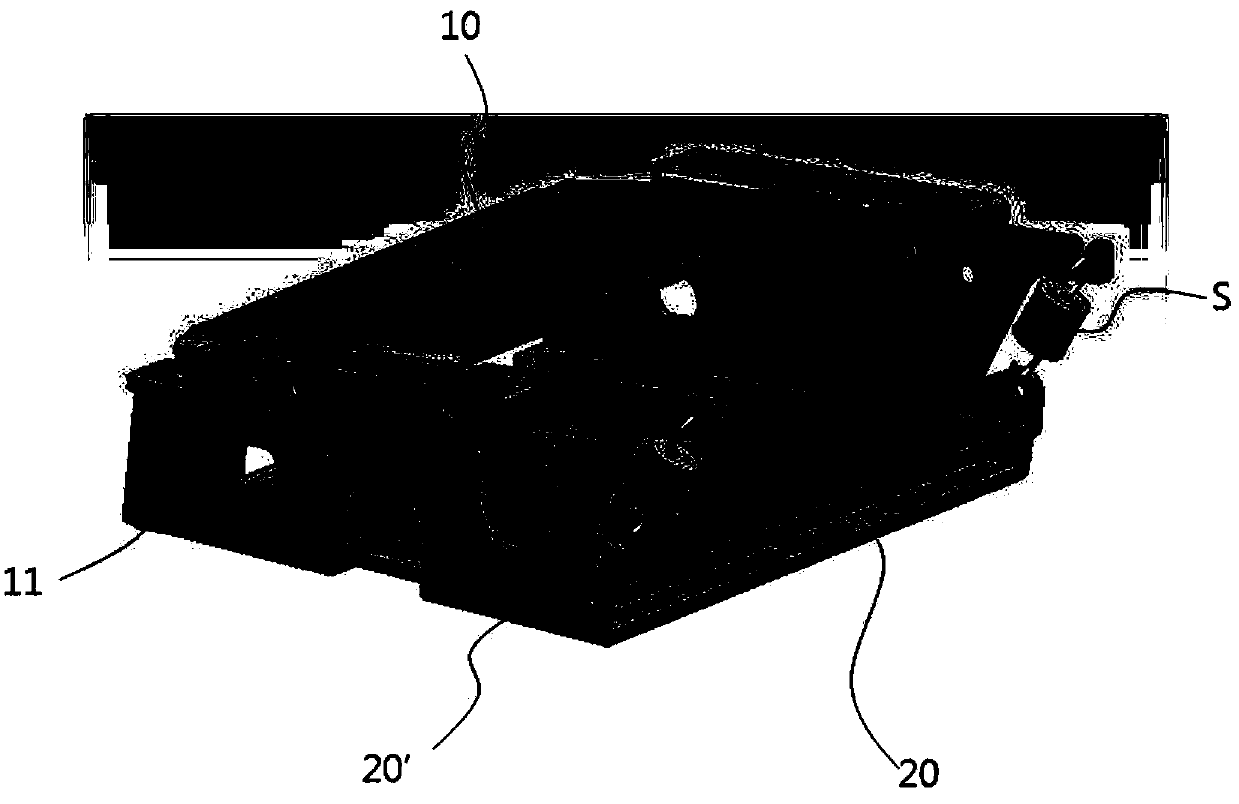
ActiveCN107639941AMinimize compressionImprove printing qualityTypewritersElectrographic process apparatusPaper sheetEngineering
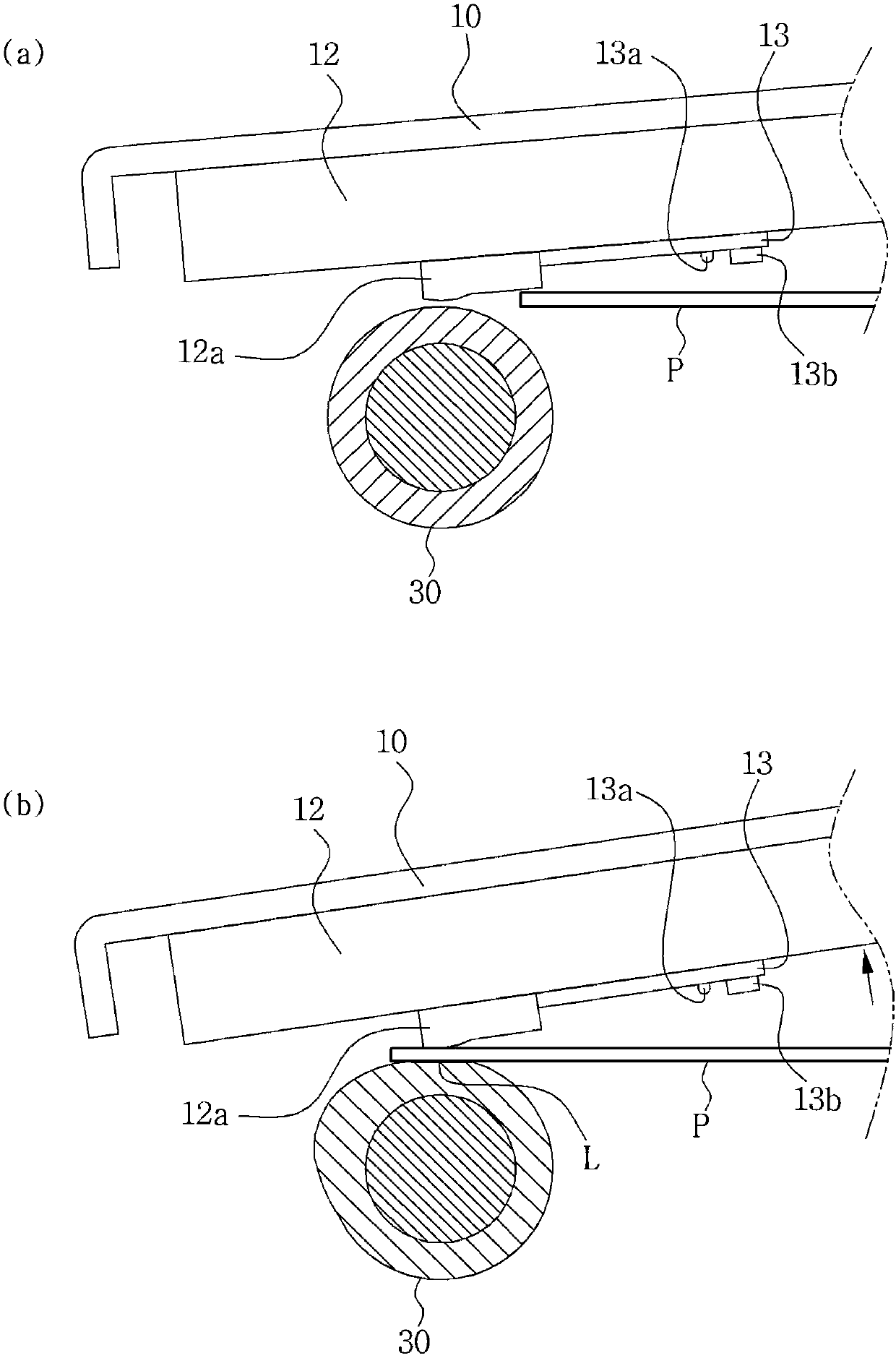
The invention relates to a photo printer. According to the photo printer of the invention, the photo printer can offer heat at given temperature to paper showing colors to thermoinduction so that theprinter can print images or texts. The photo printer including: a base adapted to accommodate paper therein; a pickup roller protruding from the bottom of the base to feed the paper accommodated in the base forwardly; a platen roller disposed in front of the base to discharge the paper fed by the pickup roller forwardly; a head located above the platen roller to apply given heat to the paper; a swing bracket having a mounting portion formed at the front side thereof in such a manner as to be coupled to the head and coupling portions formed at the rear side thereof in such a manner as to be rotatably coupled to the base to allow the head to swing; and pressurizing means adapted to pressurize the head downwardly to allow the head to come into close contact with the paper advancing into the space between the head and the platen roller.
Owner:DS GLOBAL
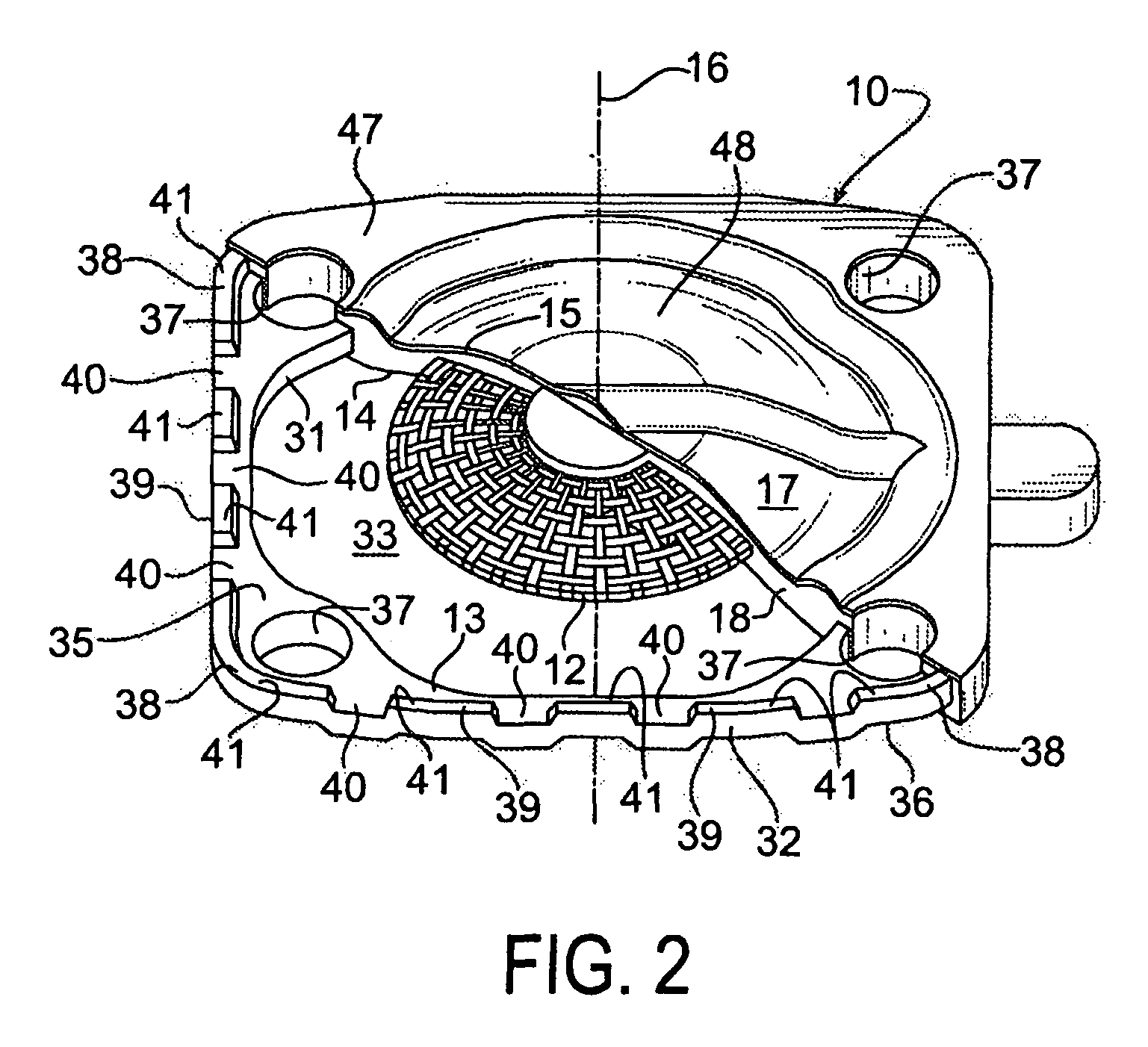
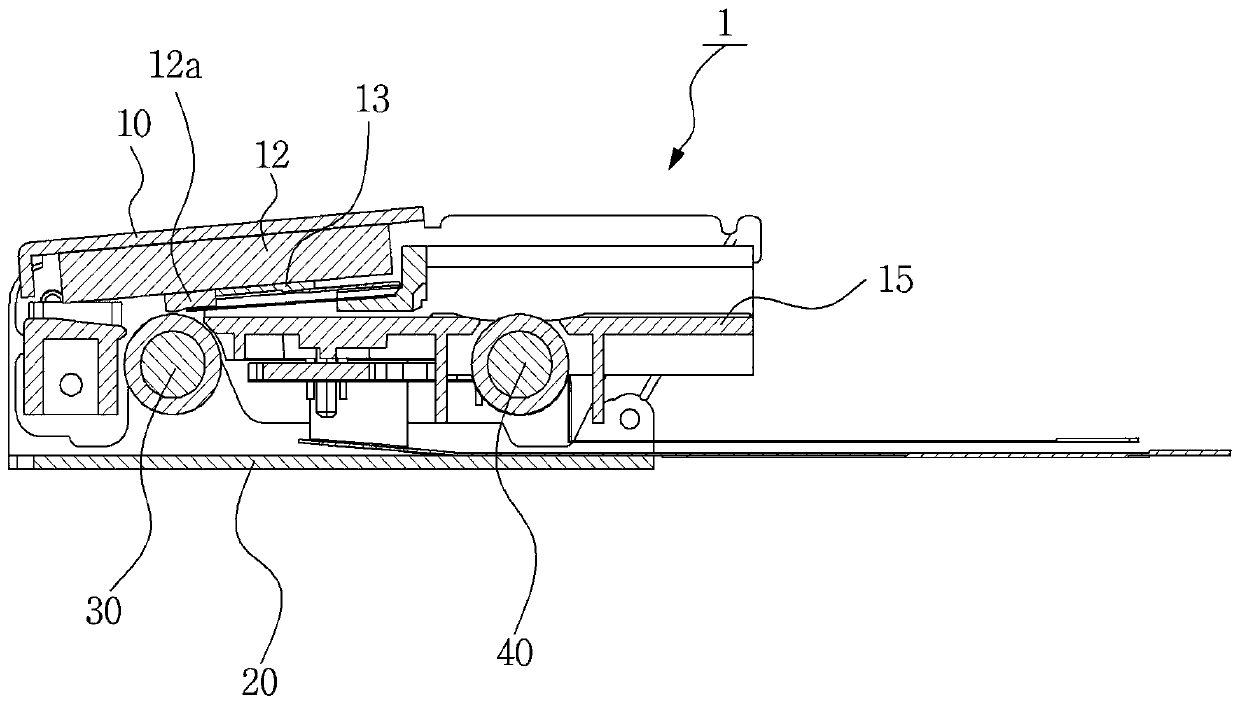
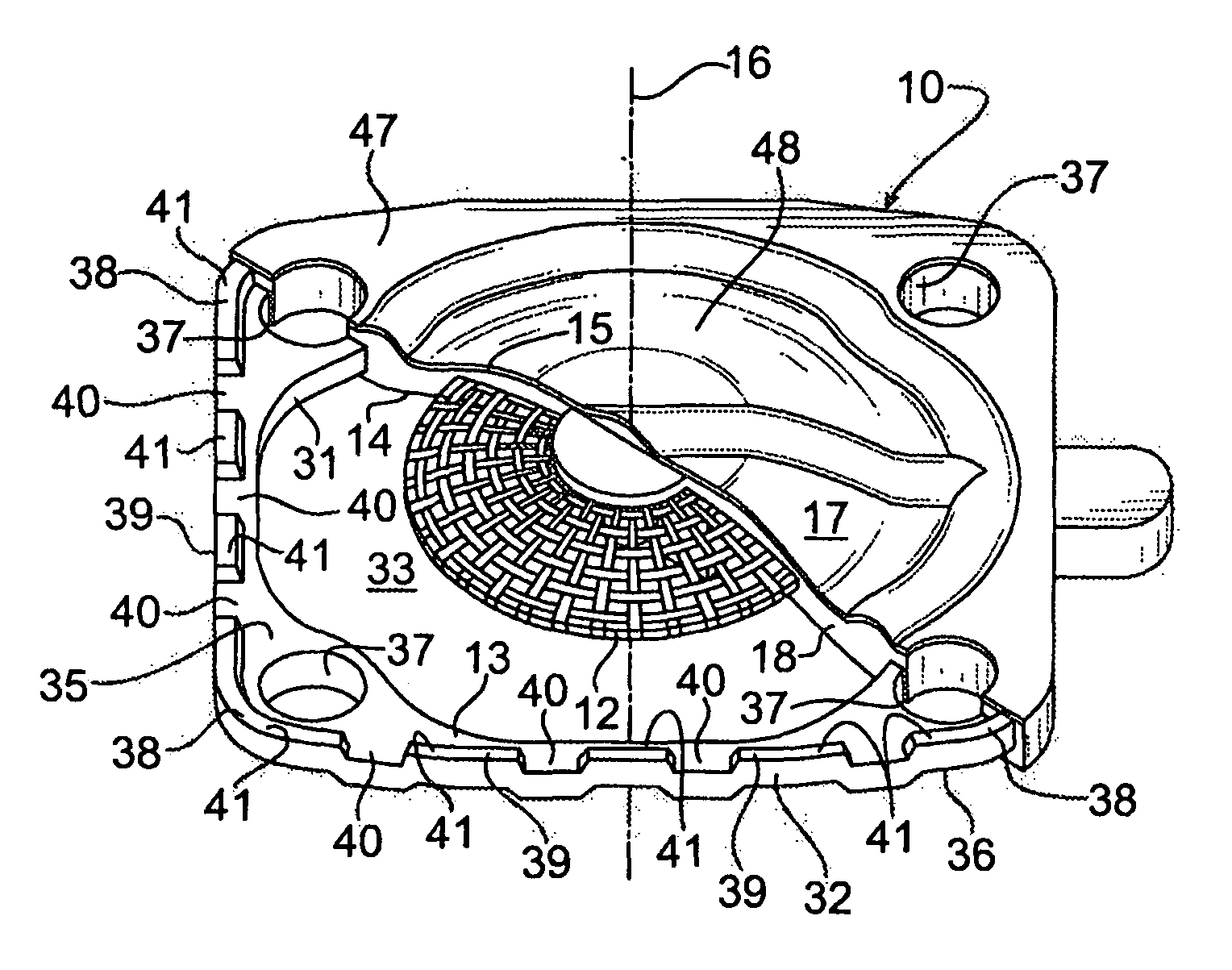
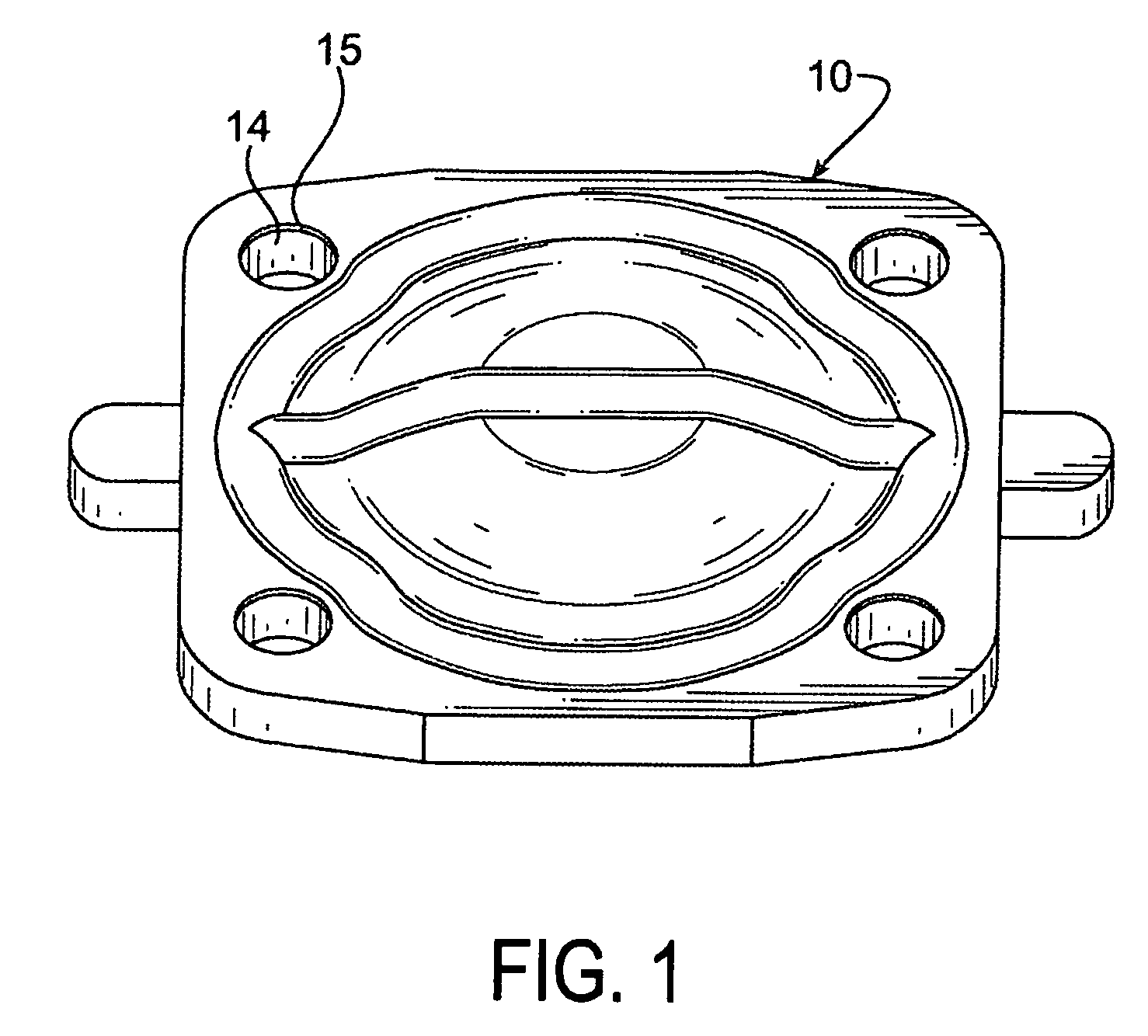
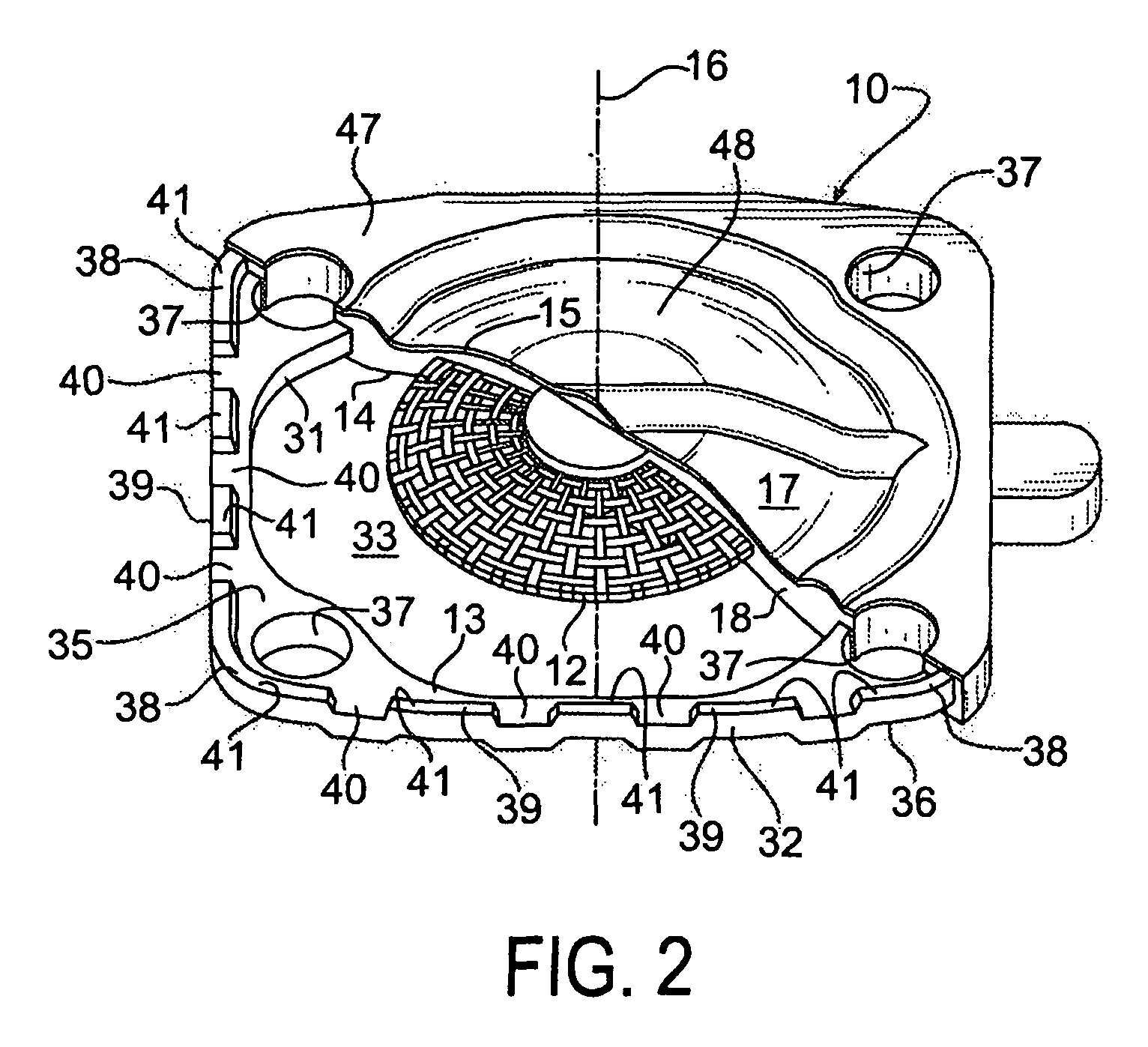
Seal assembly
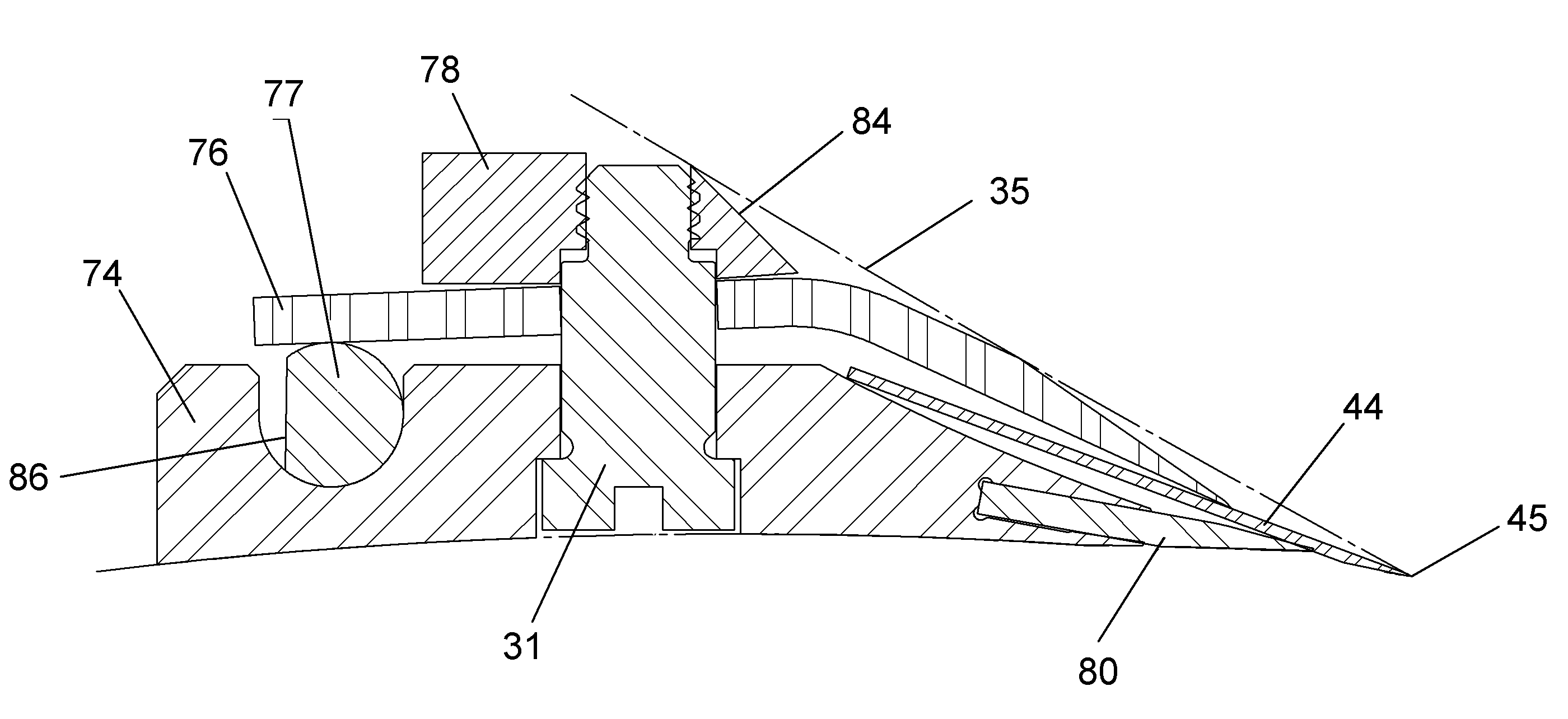
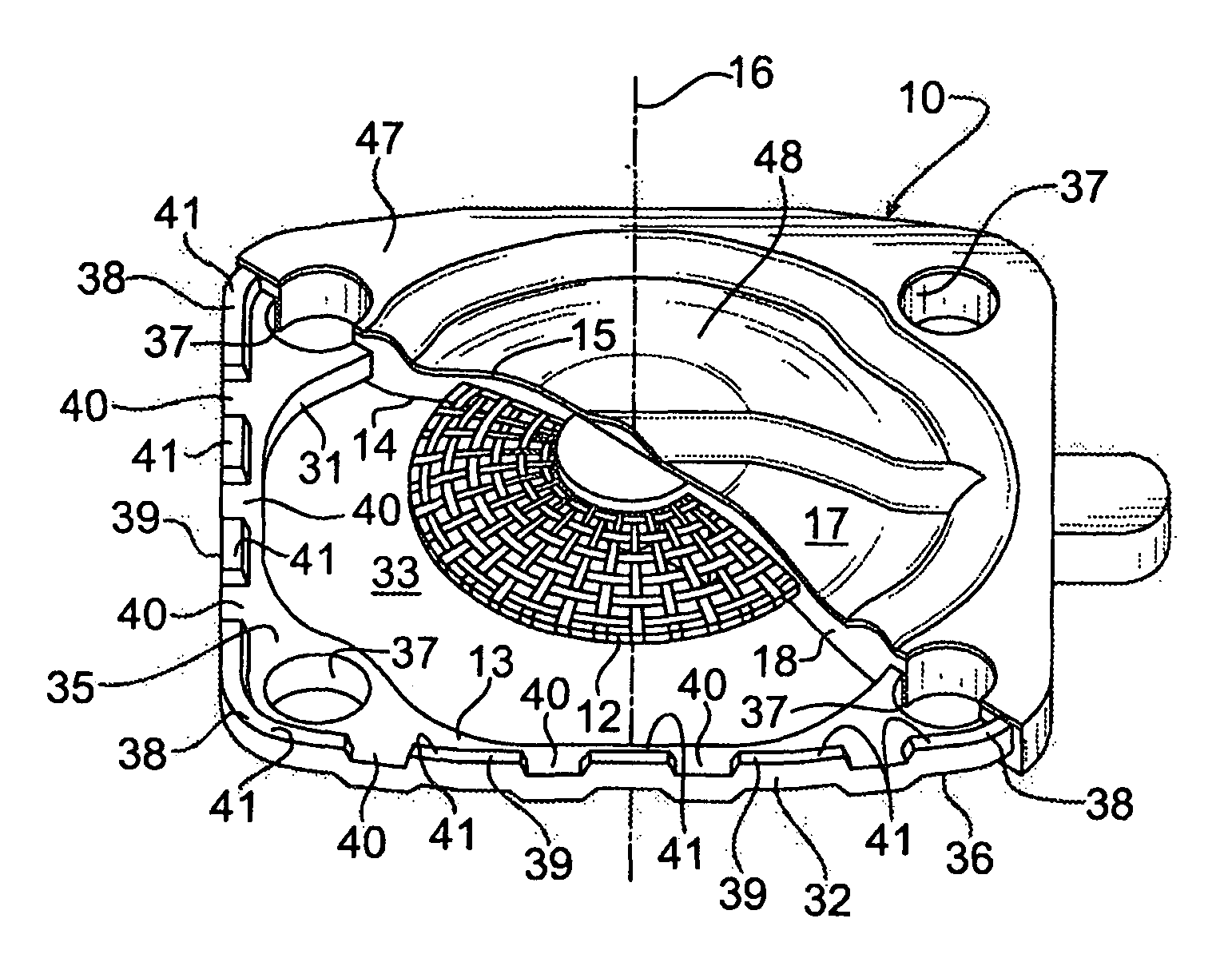
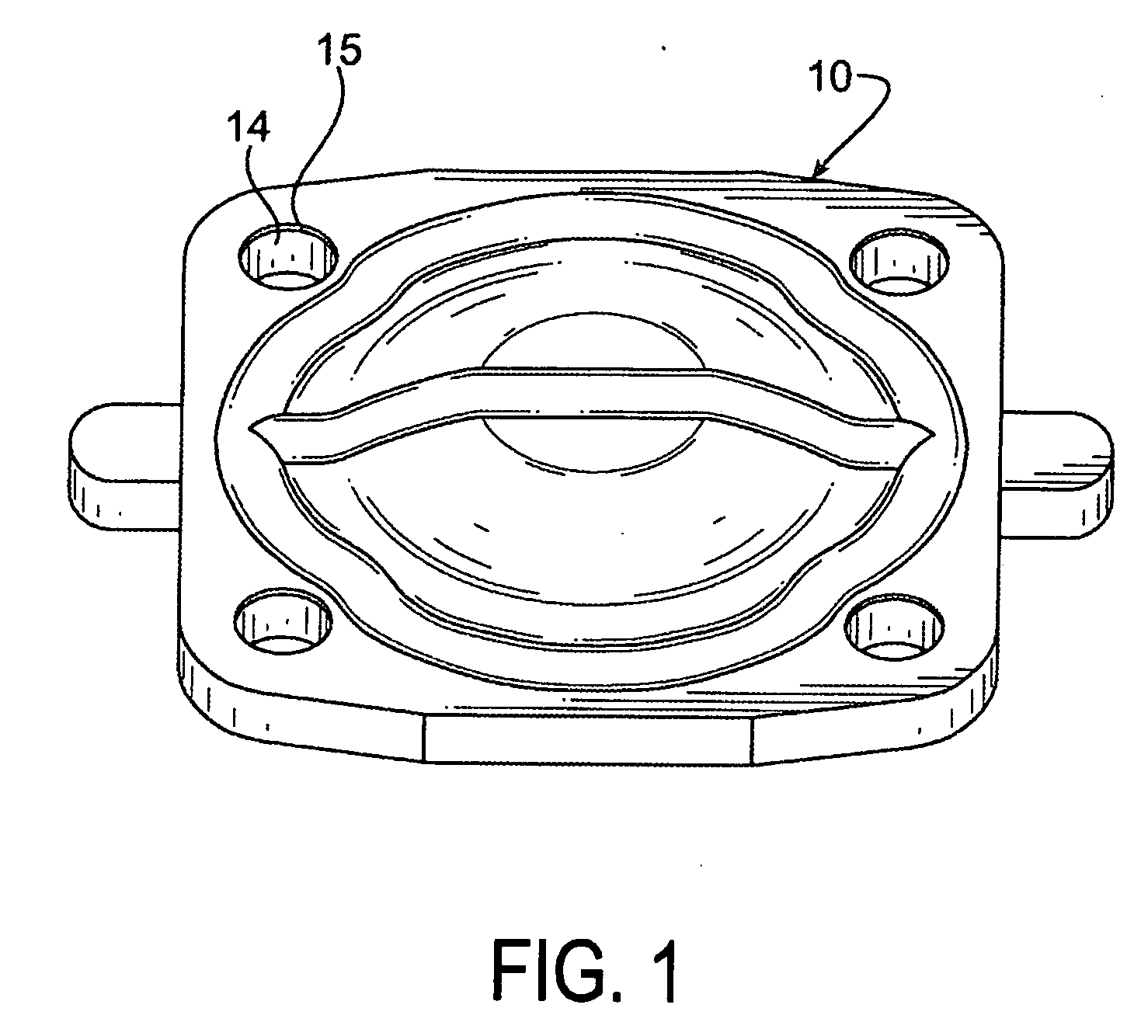
ActiveUS20120200047A1Minimize impactMinimize extrusionEngine sealsDiaphragm valvesMechanical engineeringEngineering
A seal assembly 10 includes an attachment stud 11, a reinforcing mesh 12, a rigid carrier 13, an elastomeric body 14, and an elastomeric film 15, each having a longitudinal axis 16. The carrier 13 includes an inner peripheral surface 31 defining a central opening 33 and an outer peripheral surface 32. A lateral surface 35 of the carrier 13 extends between the peripheral surfaces. Corner towers 38 and intermediate towers 39 are arranged along the outer peripheral surface 32. The towers are spaced apart by openings 40 and terminate in control surfaces 41. The body 14 includes a stationary portion 47 generally aligned with the carrier 13 and a diaphragm portion 48 generally aligned with the opening 33. The film 15 extends over and is bonded to the body portion 14 and the towers 38, 39 to provide a smooth uninterrupted chemically resistant surface for the seal assembly 10.
Owner:PARKER INTANGIBLES LLC
Photo printer
InactiveCN107639942AImprove printing qualitySimple structureTypewritersElectrographic process apparatusEngineeringMechanical engineering
Provided is a photo printer including: a base adapted to accommodate paper therein; a pickup roller protruding from the bottom of the base to feed the paper accommodated in the base forwardly; a platen roller disposed in front of the base to discharge the paper fed by the pickup roller forwardly; a head located above the platen roller to apply given heat to the paper; a swing bracket having a mounting portion formed at the front side thereof in such a manner as to be coupled to the head and coupling portions formed at the rear side thereof in such a manner as to be rotatably coupled to the base to allow the head to swing; and pressurizing means adapted to pressurize the head downwardly to allow the head to come into close contact with the paper advancing into the space between the head andthe platen roller.
Owner:DS GLOBAL
Stripping vessel for removing hydrocarbons entrained in catalyst particles
ActiveUS9446398B2Negatively impacting the flow of catalyst andMinimizes accumulationMolecular sieve catalystsCatalyst regeneration/reactivationMarine engineeringStructured packing
Owner:UOP LLC
photo printer
InactiveCN107639942BMinimize compressionIncrease contact areaTypewritersElectrographic process apparatusGear wheelElectric machinery
Provided is a photo printer including: a base adapted to accommodate paper therein; a pickup roller protruding from the bottom of the base to feed the paper accommodated in the base forwardly; a platen roller disposed in front of the base to discharge the paper fed by the pickup roller forwardly; a head located above the platen roller to apply given heat to the paper; a swing bracket having a mounting portion formed at the front side thereof in such a manner as to be coupled to the head and coupling portions formed at the rear side thereof in such a manner as to be rotatably coupled to the base to allow the head to swing; and pressurizing means adapted to pressurize the head downwardly to allow the head to come into close contact with the paper advancing into the space between the head andthe platen roller.
Owner:DS GLOBAL CO LTD
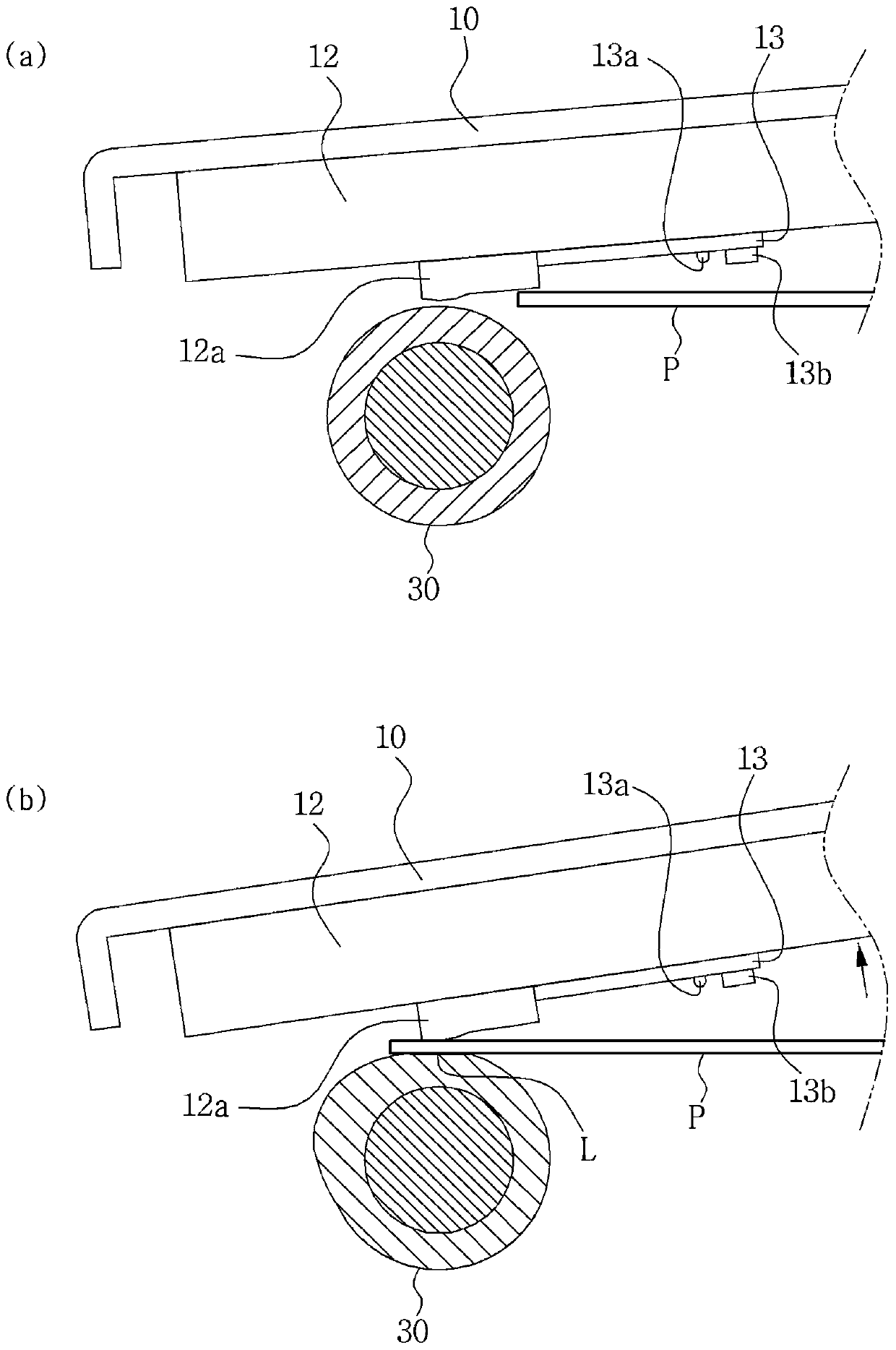
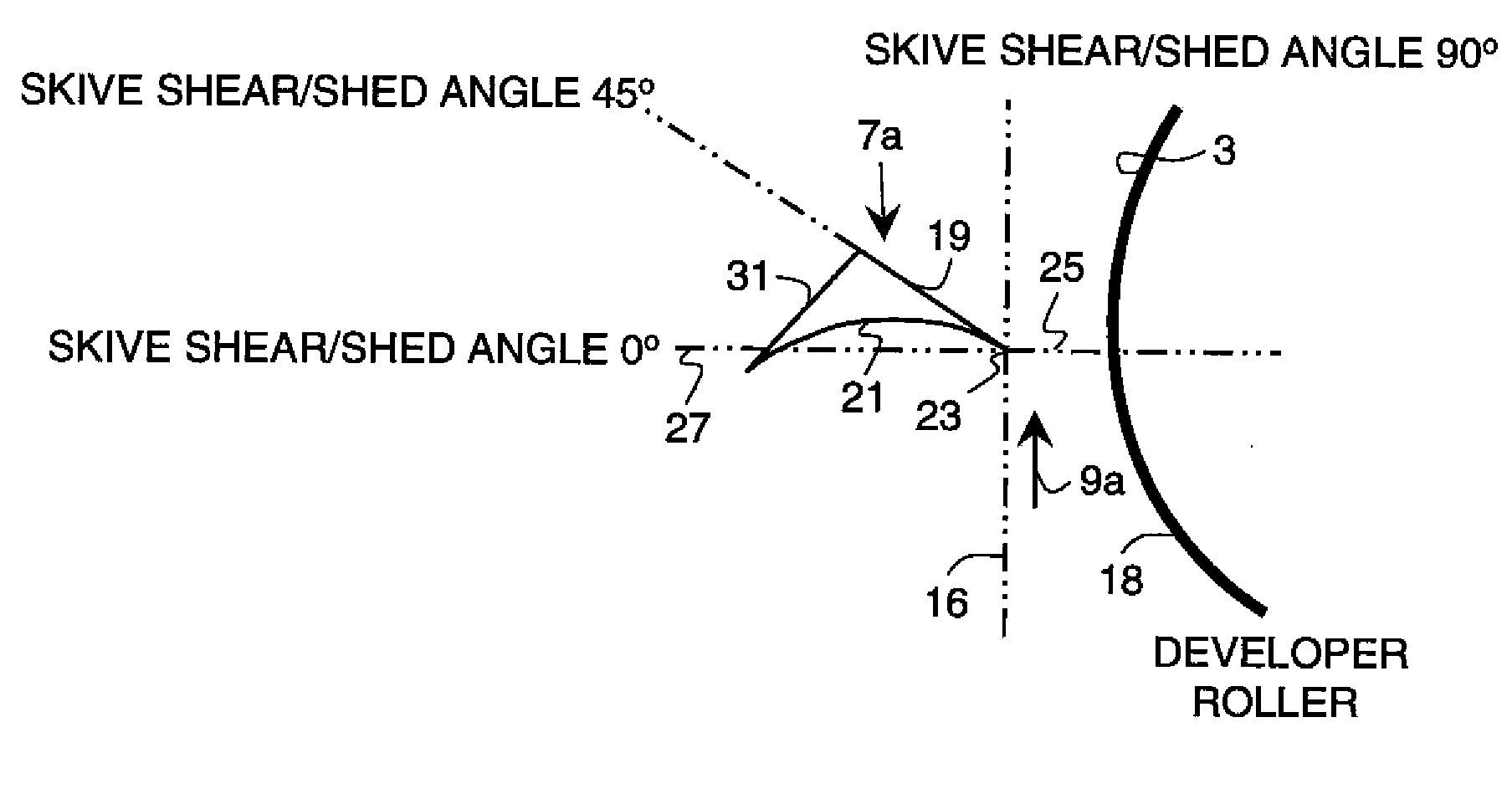
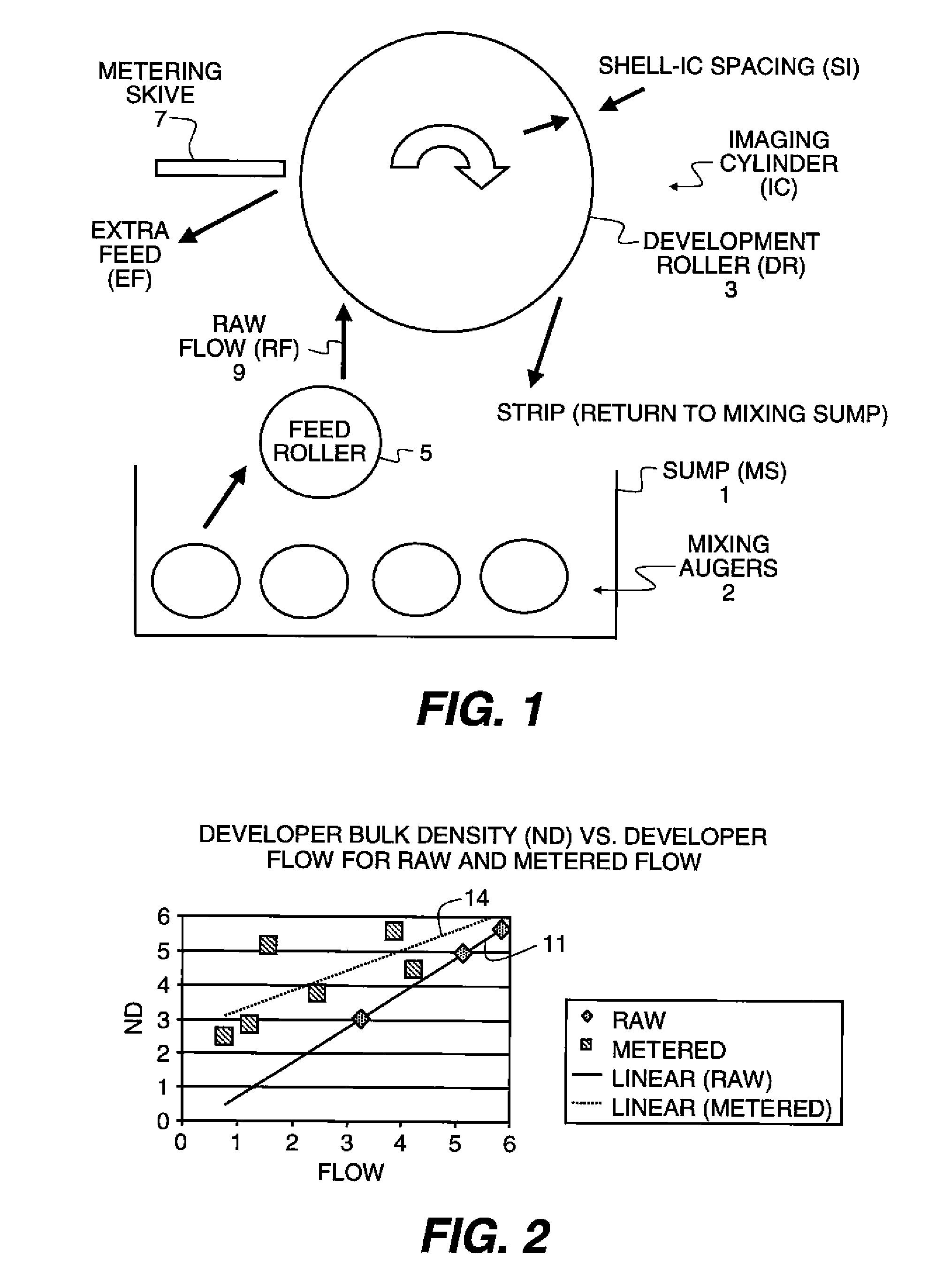
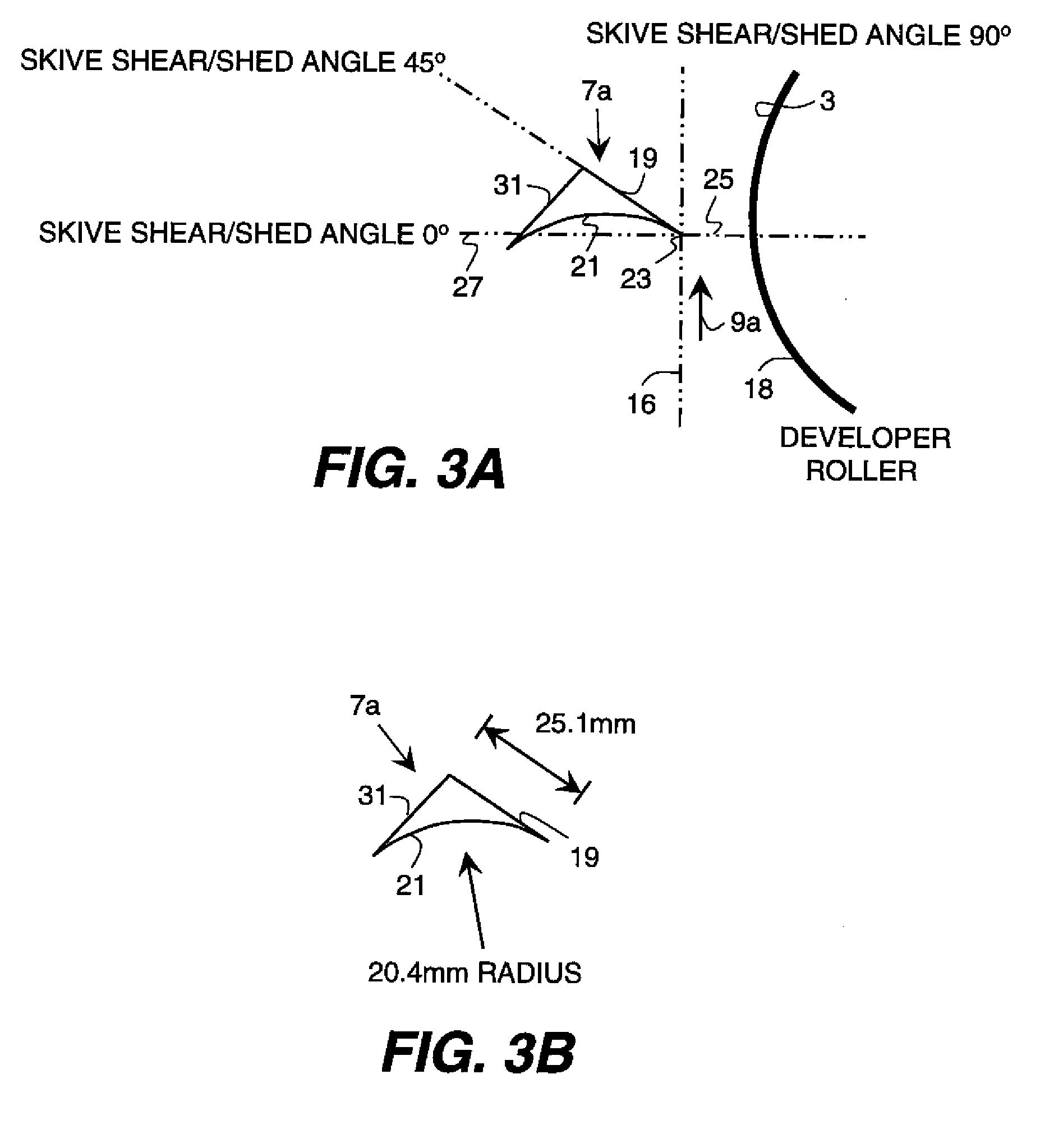
Metering skive for a developer roller
InactiveUS20100158580A1Minimize compressionPromote sheddingElectrographic process apparatusMechanical engineeringShear angle
Owner:EASTMAN KODAK CO
Breastfeeding device
InactiveUS20200060940A1Minimize compressionMammary implantsBrassieresBiomedical engineeringLactation
A breastfeeding device adapted to fit over a natural breast is provided. The device includes a reservoir that stores a received liquid and has an inner surface and an outer surface. Additionally, the device includes an access opening that is formed in the outer surface of the reservoir and is adapted to receive an attachment. The inner surface of the reservoir is attached onto a surface of the natural breast emulating a contour thereof.
Owner:CHILDRENS MEDICAL CENT CORP
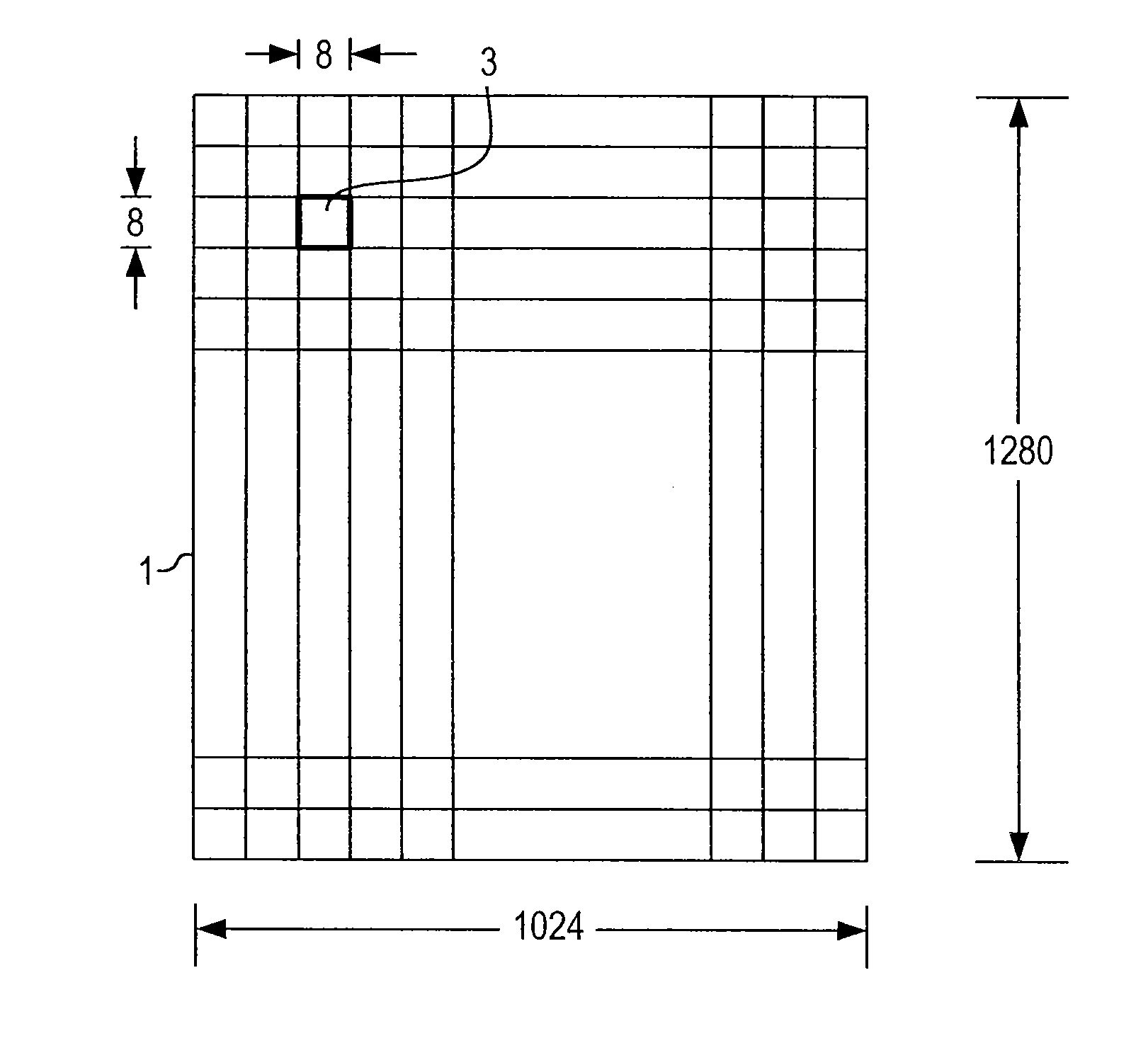
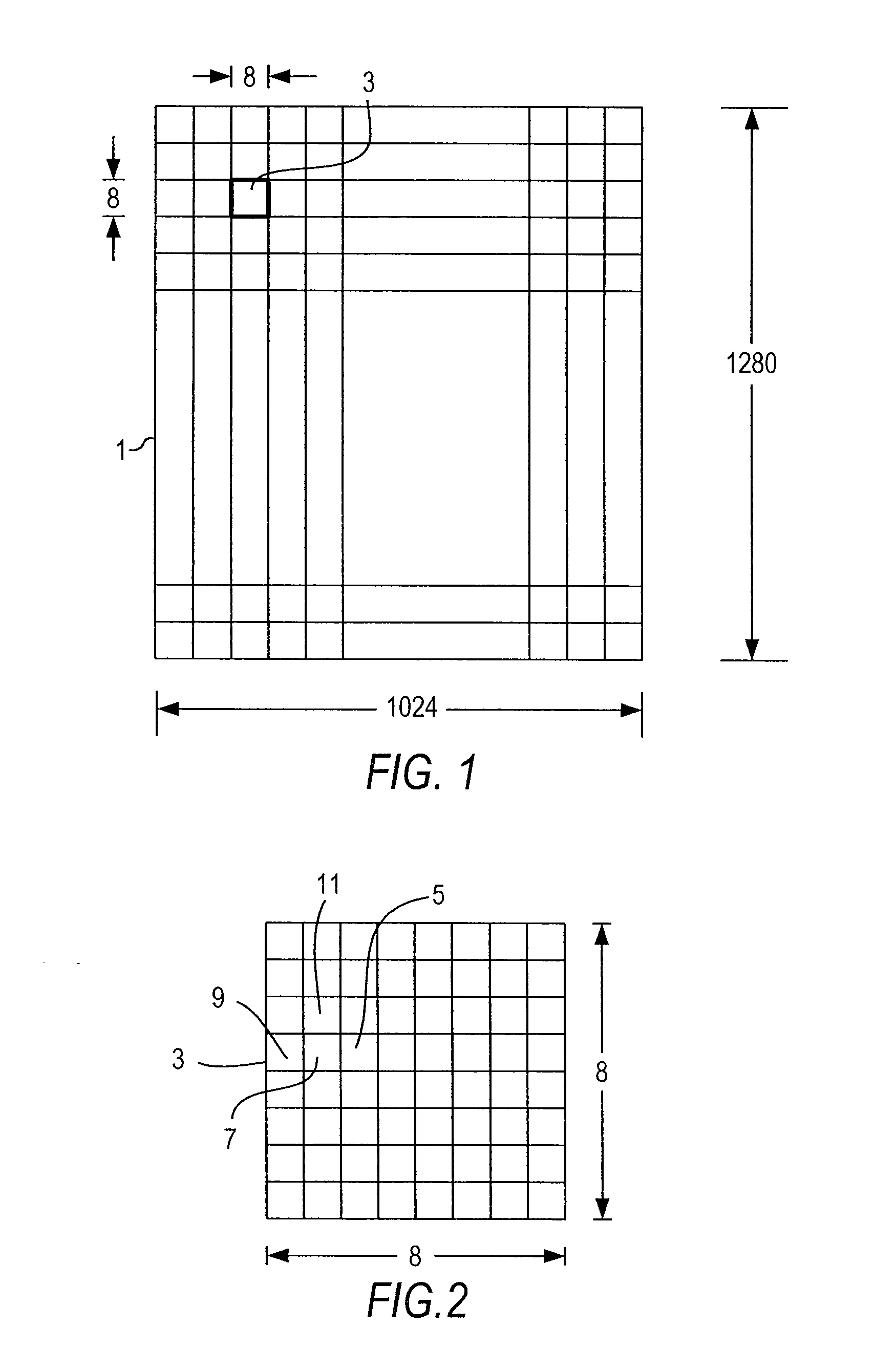
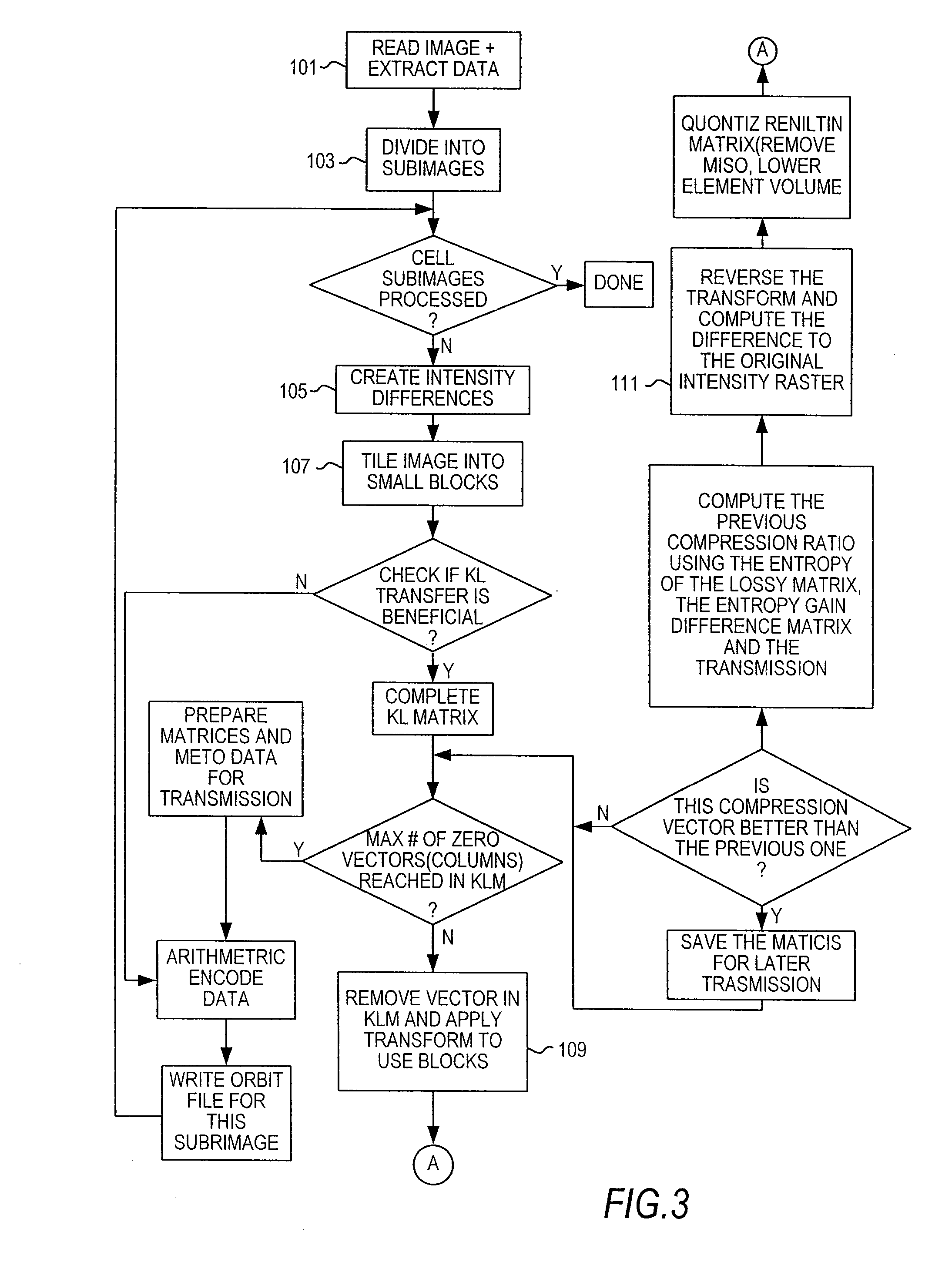
Optimized lossless data compression methods
ActiveUS20070053601A1Great freedom of choiceEasy to compressCharacter and pattern recognitionDigital video signal modificationData compressionLossless coding
A method for losslessly encoding and compressing and decoding original, raw, raster data, and other files by optimizing the results of preprocessing and transformation techniques. Four stages are involved: pre-processing during which the data is replaced by predictive values and the deviations from the predictive value; mapping each block through a transform sequence using a lossy reversible mapping; minimizing the joint entropy of the transformed data and deviation data by varying parameters for the predictive and transform step; and encoding the transformed sequence. At each stage of the compression of the data, different techniques are tried and compared and an optimal technique used to carry out that state.
Owner:PIXSPAN
Seal assembly
InactiveUS9109707B2Minimize extrusionImproved compression set propertiesDiaphragm valvesEngine sealsMechanical engineering
A seal assembly 10 includes an attachment stud 11, a reinforcing mesh 12, a rigid carrier 13, an elastomeric body 14, and an elastomeric film 15, each having a longitudinal axis 16. The carrier 13 includes an inner peripheral surface 31 defining a central opening 33 and an outer peripheral surface 32. A lateral surface 35 of the carrier 13 extends between the peripheral surfaces. Corner towers 38 and intermediate towers 39 are arranged along the outer peripheral surface 32. The towers are spaced apart by openings 40 and terminate in control surfaces 41. The body 14 includes a stationary portion 47 generally aligned with the carrier 13 and a diaphragm portion 48 generally aligned with the opening 33. The film 15 extends over and is bonded to the body portion 14 and the towers 38, 39 to provide a smooth uninterrupted chemically resistant surface for the seal assembly 10.
Owner:PARKER INTANGIBLES LLC
Side-loading ratchet device
ActiveUS20140338161A1Avoid lateral loadMinimize compressionSnap fastenersClothes buttonsEngineeringMechanical engineering
A ratchet device 1 for a strap has a body 105 having a side member 109, and a spool 119 having a side 119a rotatably supported by the side member and a ratchet wheel 121 fixed thereto. The body has a member 161 moveable between an open position in which the other side 119b of the spool is exposed to enable a strap to be laterally loaded into the spool 119 from the exposed side of the spool and laterally unloaded from the spool, and a closed position in which said lateral loading and unloading are prevented.
Owner:ARMOR HLDG
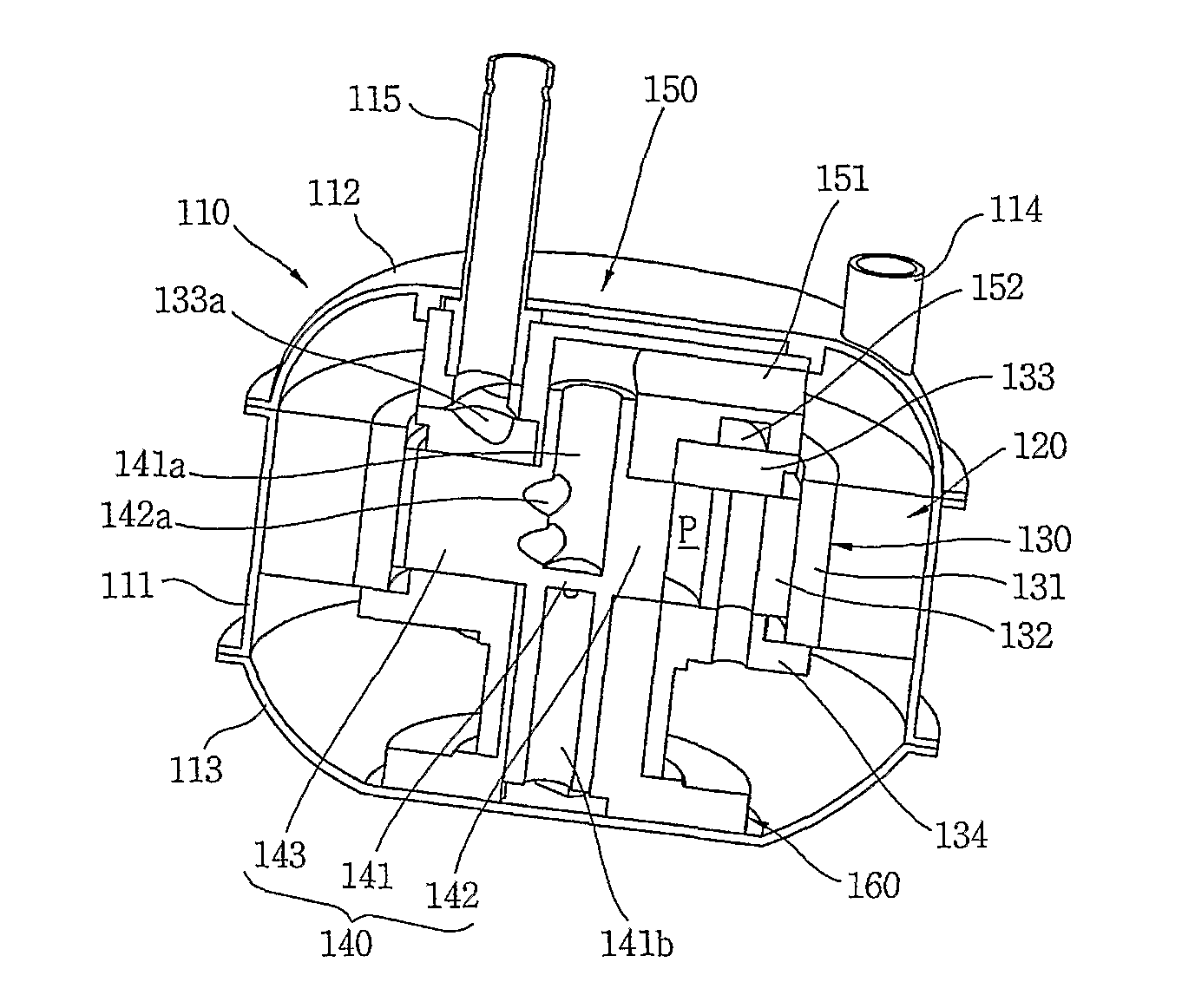
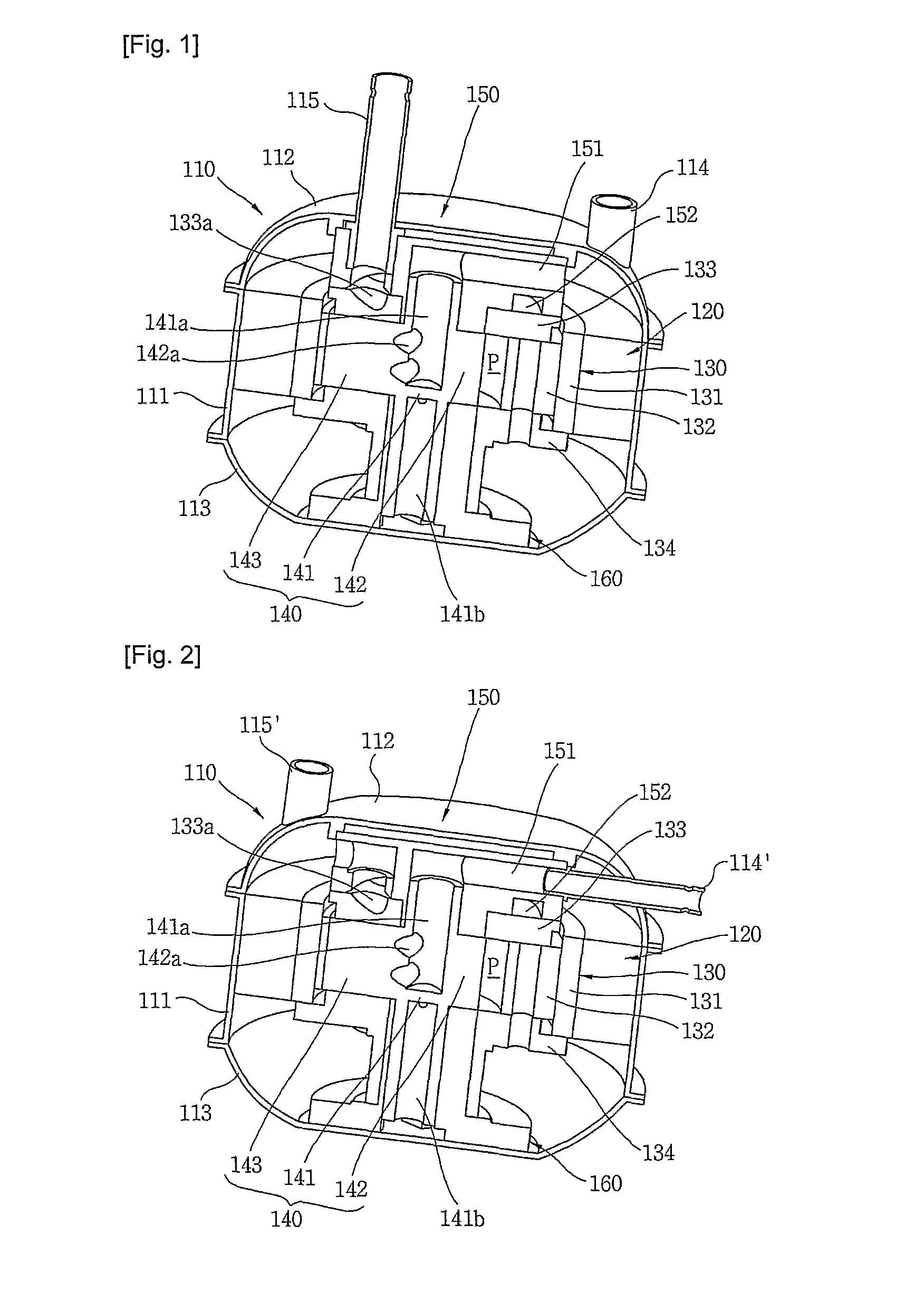
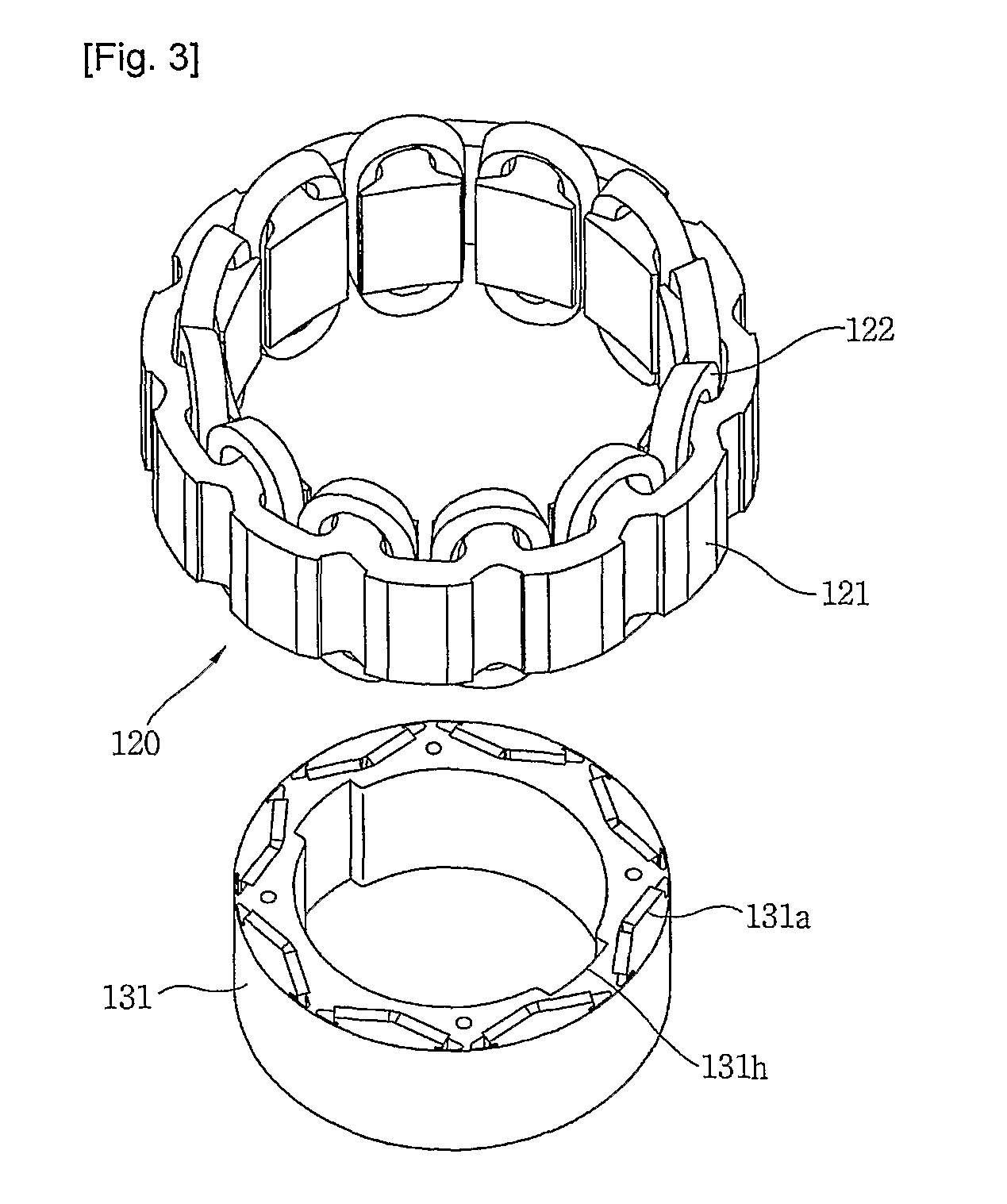
Compressor
InactiveUS20110120178A1Minimal heightSmall sizeRotary/oscillating piston combinations for elastic fluidsSealing arrangement for pumpsElectromagnetic fieldRefrigerant
The present invention provides a compressor, comprising a stator (120); a cylinder type rotor (131) rotating within the stator (120) by a rotating electromagnetic field from the stator (120), with the rotor defining a compression chamber inside; a roller (142) rotating within the compression chamber of the cylinder type rotor (131) by a rotational force transferred from the rotor (131), with the roller (142) compressing refrigerant during rotation; a vane (146) dividing the compression chamber into a suction region where refrigerant is sucked in and a compression region where the refrigerant is compressed / discharged from, with the vane (143) transferring the rotational force from the cylinder type rotor (131) to the roller (142); an axis of rotation (141) integrally extended from the roller (142) in an axial direction; and a suction passage (141a) sucking refrigerant into the compression chamber through the axis of rotation and the roller.
Owner:LG ELECTRONICS INC
Clot retrieval device for removing occlusive clot from a blood vessel
ActiveUS11147572B2Prevent distal egressReduce radial forceDiagnosticsExcision instrumentsEngineeringSurgery
A clot retrieval device for removing occlusive clot from a blood vessel. The device comprises an inner elongate body having a collapsed delivery configuration and an expanded deployed configuration. An outer elongate body is at least partially overlying the inner elongate body. The outer elongate body is expandable to a radial extent which is greater than the radial extent of the inner body in the deployed configuration to define a clot reception space. The outer elongate body has a plurality of clot receiving openings and a plurality of clot engaging regions. The clot engaging regions are adapted, on engagement with clot, to urge clot towards the clot receiving openings and into the reception space between the outer elongate body and the inner elongate body, wherein the radial force profile of the device varies along the length of the device.
Owner:NEURAVI
Weight-bearing lower extremity brace
InactiveUS20160287410A1Reduce and eliminate tourniquet effectMinimize compressionArtificial legsEngineeringCuff
Owner:TOAD MEDICAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com