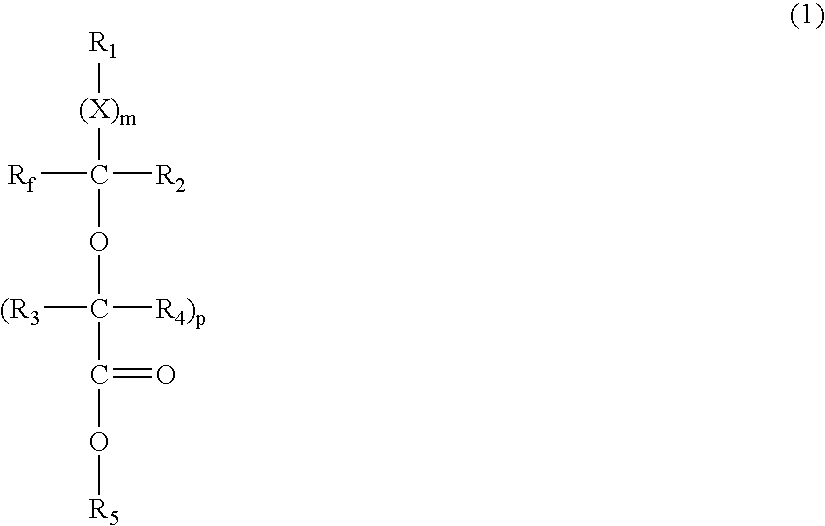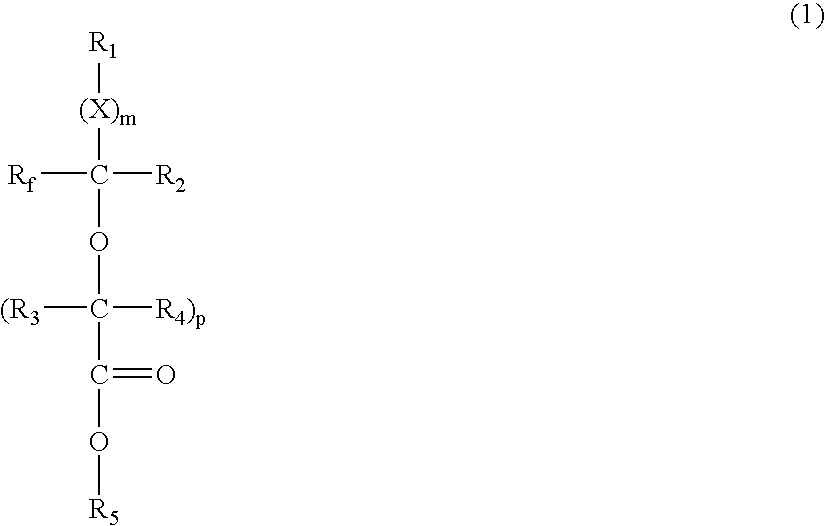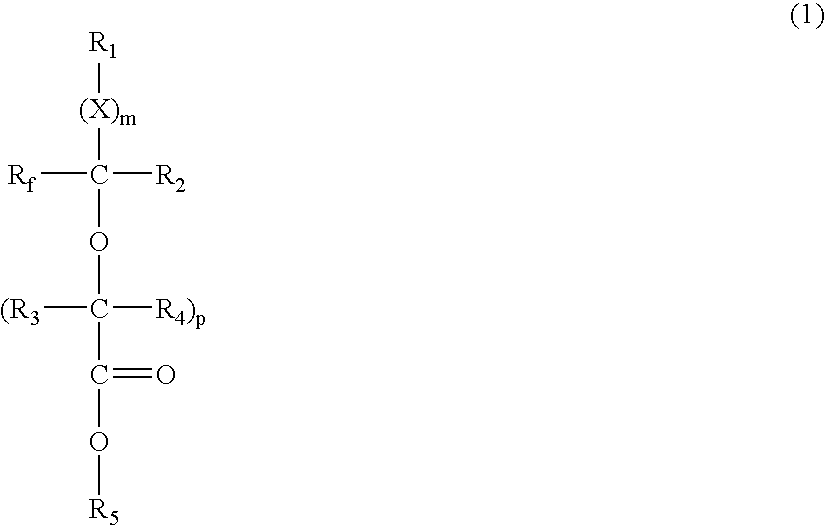Photoresist composition for deep ultraviolet lithography
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0090] Table 1 shows the TGA onset of deprotection of MOM and t-boc compared to that found for the deprotection with the BOCME group on representative examples of polymers (FIG. 6) belonging to the two classes of cyclic fluoroalcohol bearing polymer described earlier.
1TABLE 1 Onset of deprotection of polymers I and II with various protecting groups* Protecting Polymer Onset of group (% protection) Deprotection (.degree. C.) BOCME II (30%) 174 BOCME I (30%) 206 T-BOC I (30%) 120 MOM II (20%) <100 *Data gathered at 20.degree. C. / min heating rate
[0091] Table 1 clearly shows that polymers with the BOCME group thermally deprotect at a higher temperature than the same polymer with the t-boc and MOM groups
example 2
[0092] The contrasts of resists were measured by coating them at a thickness of 1350 A (Angstroms) and after exposure using an open frame reticle baking and developing the film and measuring the normalized thickness as a function of dose. The contrast is taken from the slope of plot of normalized thickness versus log(dose). Processing conditions were as follows:
[0093] The exposures were done an Exitech 157 nm small field (1.5.sub.--1.5 mm2) mini-stepper (0.6 NA) using open frame exposure reticle at International SEMATECH in Austin. An FSI Polaris 2000 track was used to coat, bake, and develop the resist films. A Prometrix interferometer was used to measure resist thickness.
[0094] The photoresist formulations preparation and resultant contrasts are as follows:
[0095] By mixing the following dry ingredients poly(tert-Butyl Bicyclo[2.2.1]hept-5-ene-2-carboxylate-co-1,1,1-trifluoro-2-(trifluoromet-hyl)pent-4-en-2-3-(Bicyclo[2.2.1]hept-5-en-2-yl)-1,1,1-trifluoro-2-(triflu-oromethyl)propan...
example 3
Synthesis of BOCME Protected Poly(3-(bicyclo[2.2.1]hept-5-en-2-yl)-1,1,1-t-rifluoro-2-(trifluoromethyl)propan-2-ol) Using t-BuOK
[0096] Poly(3-(bicyclo[2.2.1]hept-5-en-2-yl)-1,1,1-trifluoro-2-(trifluorom-ethyl)propan-2-ol) (PBHTTP) (4.0 g, 14.59 mmol) was dissolved into 15 ml of tetrahydrofuran (THF), and solid t-BuOK (0.491 g, 4.38 mmol) was added while stirring. After 30 minutes, t-butyl bromoacetate (1.71 g, 8.76 mmol) was added to this reaction solution which was stirred for 16 hours at 25.degree. C. After removal of the solvent using a rotary evaporator, the resultant residue was dissolved in 20 ml of methanol (MeOH) containing 1.0 g of concentrated HCl. This solution was then precipitated in 180 ml of water-methanol (8:1). The polymer was isolated by filtration and further purified by dissolving it into MeOH and re-precipitating it in the water-methanol mixture. The final precipitate was then filtered, washed with water and dried overnight under vacuum (25" Hg) at 55.degree. C....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com