Devices and methods for the performance of miniaturized in vitro amplification assays
a technology of miniaturization and amplification assays, applied in the field of micro-miniaturization assays, can solve the problems of inability to achieve the desired final application of dna, requiring a complex set of devices and skilled operators, and difficulty in designing systems for moving fluids on the microchip through channels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Sample Preparation and PCR of Genomic DNA from Bovine Blood
[0221] The experiment set forth in Example 1 was repeated using whole blood samples. In these experiments, heparinized bovine blood was diluted 1:40 in deionized water. While the precise number of white blood cells (WBCs) of the bovine blood was not determined, it is known that the average value is around 5.times.10.sup.6 cells / mL.
[0222] The alkaline cell lysis solution, neutralization solution, and PCR reagents used were as described above. To the PCR reaction mixture was added the primer pair of interest, those defining the 289 bp codon for .beta.-actin.
4 .beta.-actin-F sequence: 5'-ACCCACACTGTGCCCATCTA-3' (SEQ ID No.: 5) .beta.-actin-R sequence: 5'-CGGAACCGCTCATTGCC-3'. (SEQ ID No.: 6)
[0223] The general protocol used was similar to that described above. Because white blood cells are less robust than bacteria, the lysis temperature chosen was 91.degree. C.
[0224] The rotational profile used is as described in Example 1.
[022...
example 3
Sample Preparation and PCR of pTrcHis a Plasmid DNA from E. coli
[0227] The platform shown in FIG. 14 was used for the processing of samples of E. coli and the isolation and purification of plasmid DNA. The instrument used for control of the rotational profile and thermal cycling was as described The instrument used for controlling the rotational profile and thermal cycling consisted of a personal computer, interface electronics between the PC and a servo-controlled drive motor and interface electronics between the PC and the screen-printed circuit. For this example, the spindle is driven by a servo-controlled DC motor with encoder (Micromo part # 3557K012CR). A serial port converter U. R. Kerr part # Z232-485) and motor control board U. R. Kerr, PIC-SERVO) provide a communication interface between the PC and motor. A slip-ring (Litton part # AC6023-24) provided the electrical connection between the rotating platform and the stationary control system.
[0228] The microfluidics disc was...
example 4
PCR of Genomic DNA from E. coli
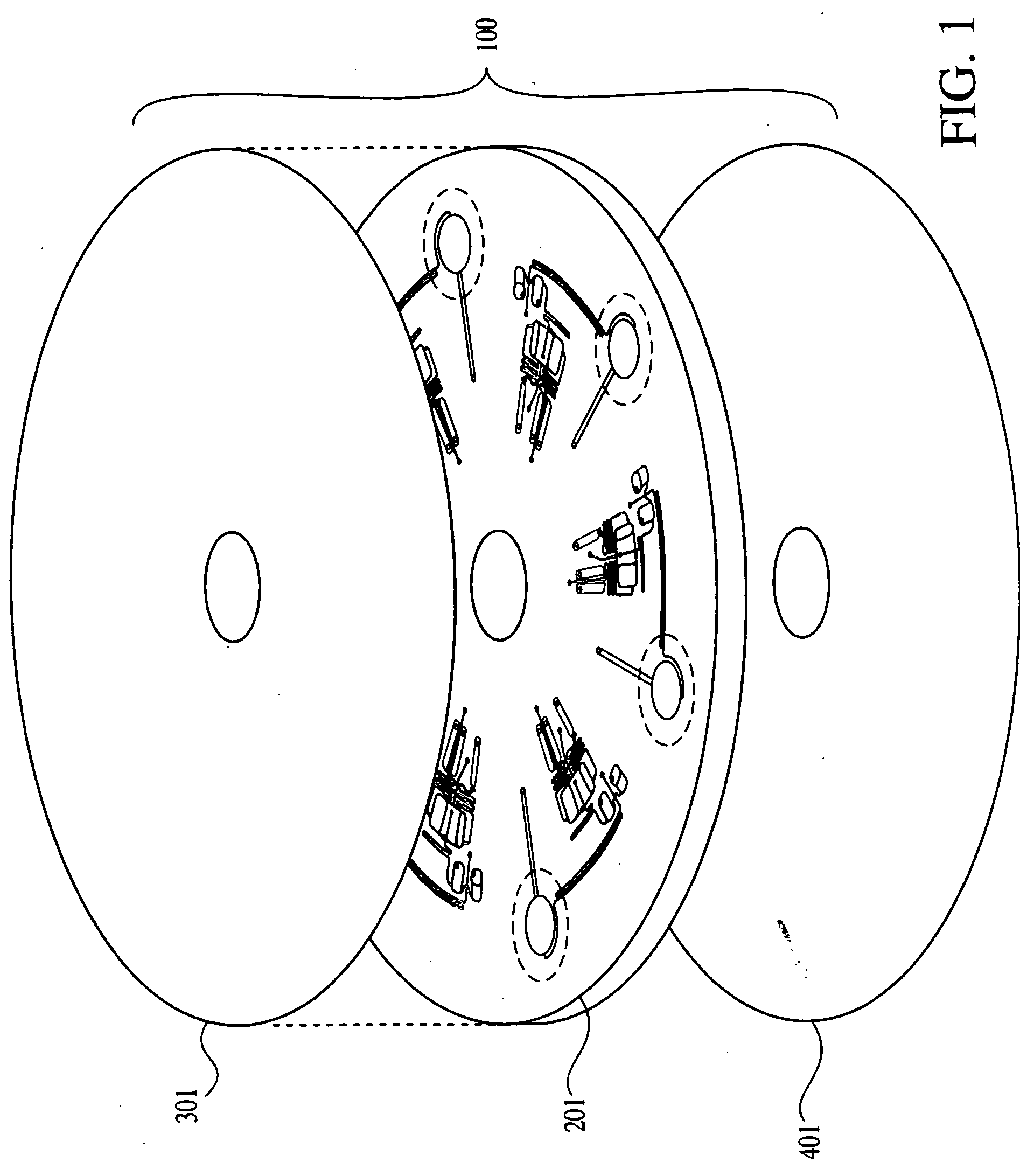
[0259] A microfluidics platform as depicted in FIGS. 6, 7, 24, 25 and 26 was used to amplify a DNA target from samples of E. coli.
[0260] FIG. 6 gives an exploded view of the two main components of the microfluidics platform. A machined fluidics disk 701 is bonded to a screen printed electrical circuit disk 710.
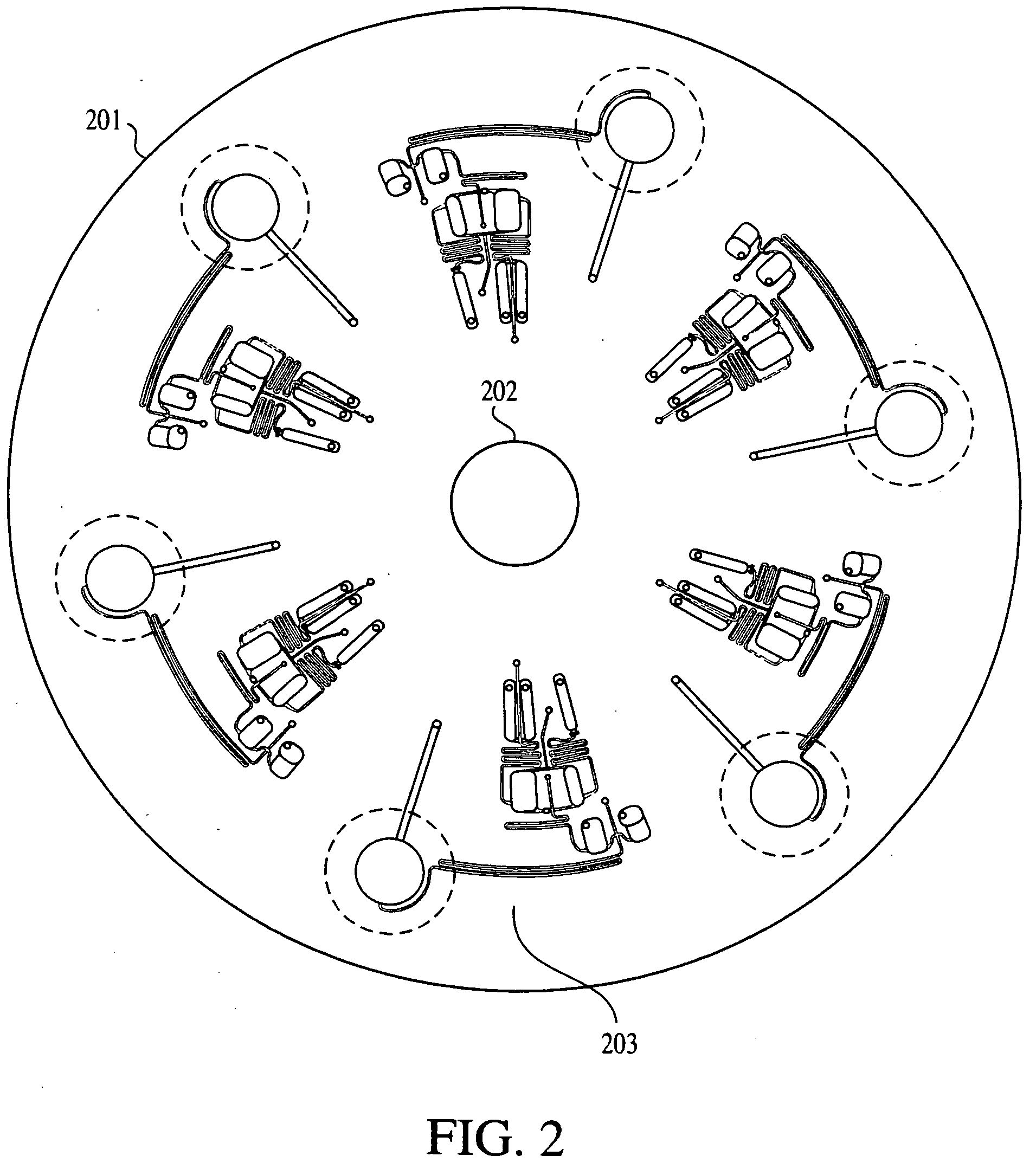
[0261] FIG. 7 shows an array of eight cycling chambers 703 within the fluidics disk. The thermal cycling chambers are circular with a diameter of 7 mm and depth of 0.5 mm. Each chamber has a reaction mixture loading channel 704 and an air channel 702. Both channels are 0.5 mm wide by 0.5 mm deep. The air channel helps to prevent liquid loss upon heating, as vapors cool and condense along its walk before spinning back down to the reaction chamber.
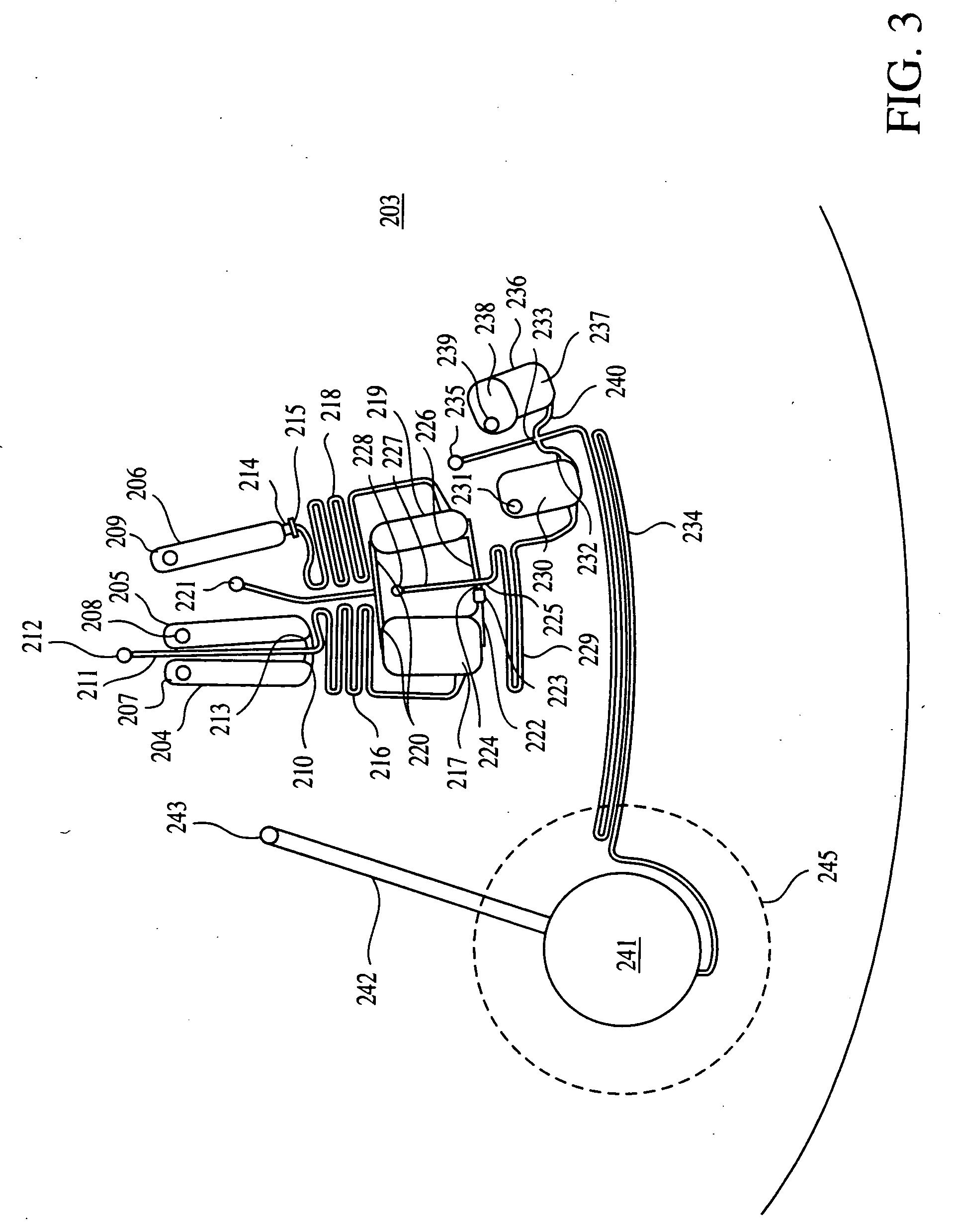
[0262] FIG. 24 shows how resistive heaters are arrayed on the electrical circuit disk. The resistive heater patches 713 are squares, 10 mm on a side. The heater dimensions were chosen to be larger than th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameters | aaaaa | aaaaa |
| contact angle | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com