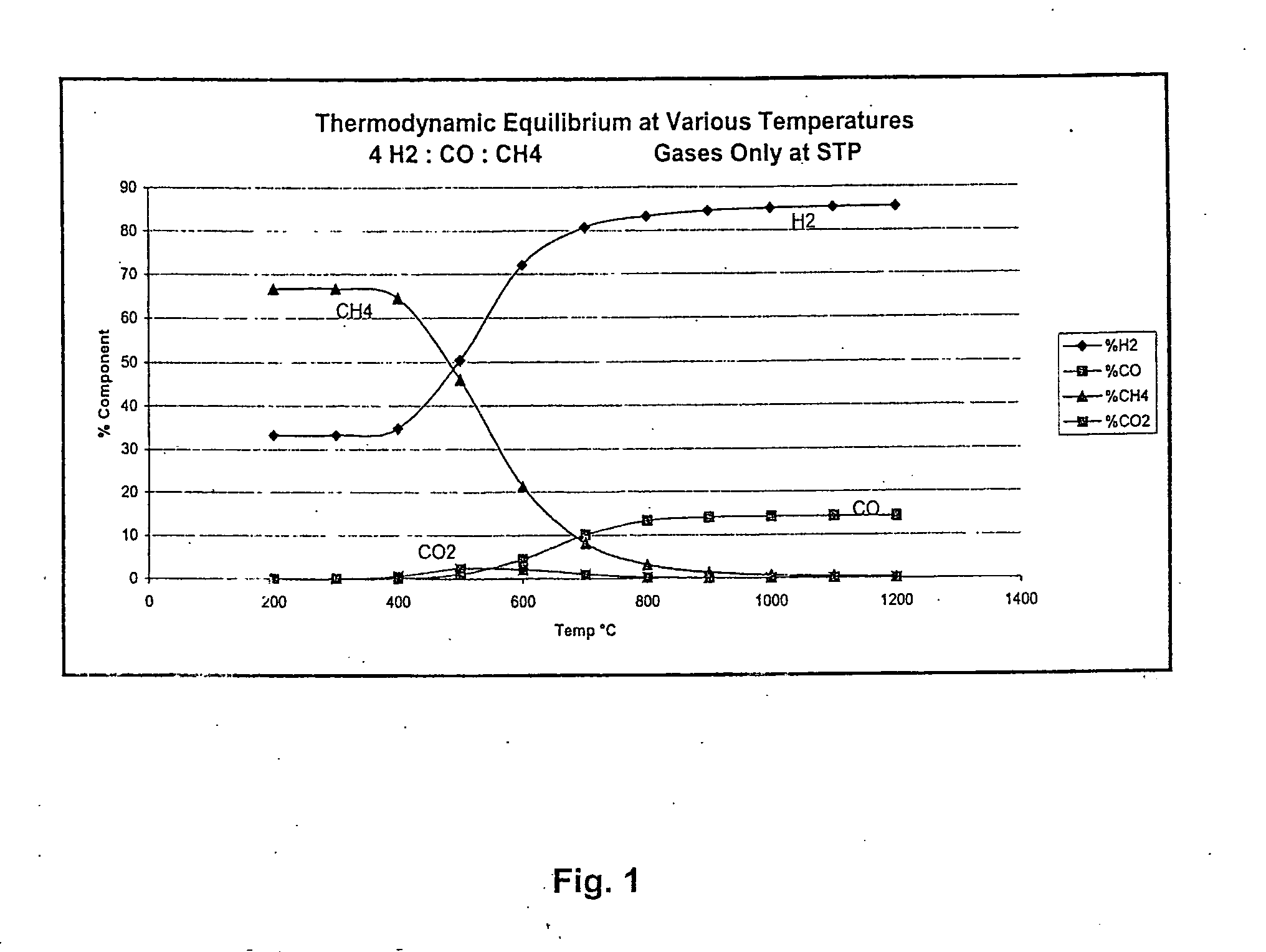
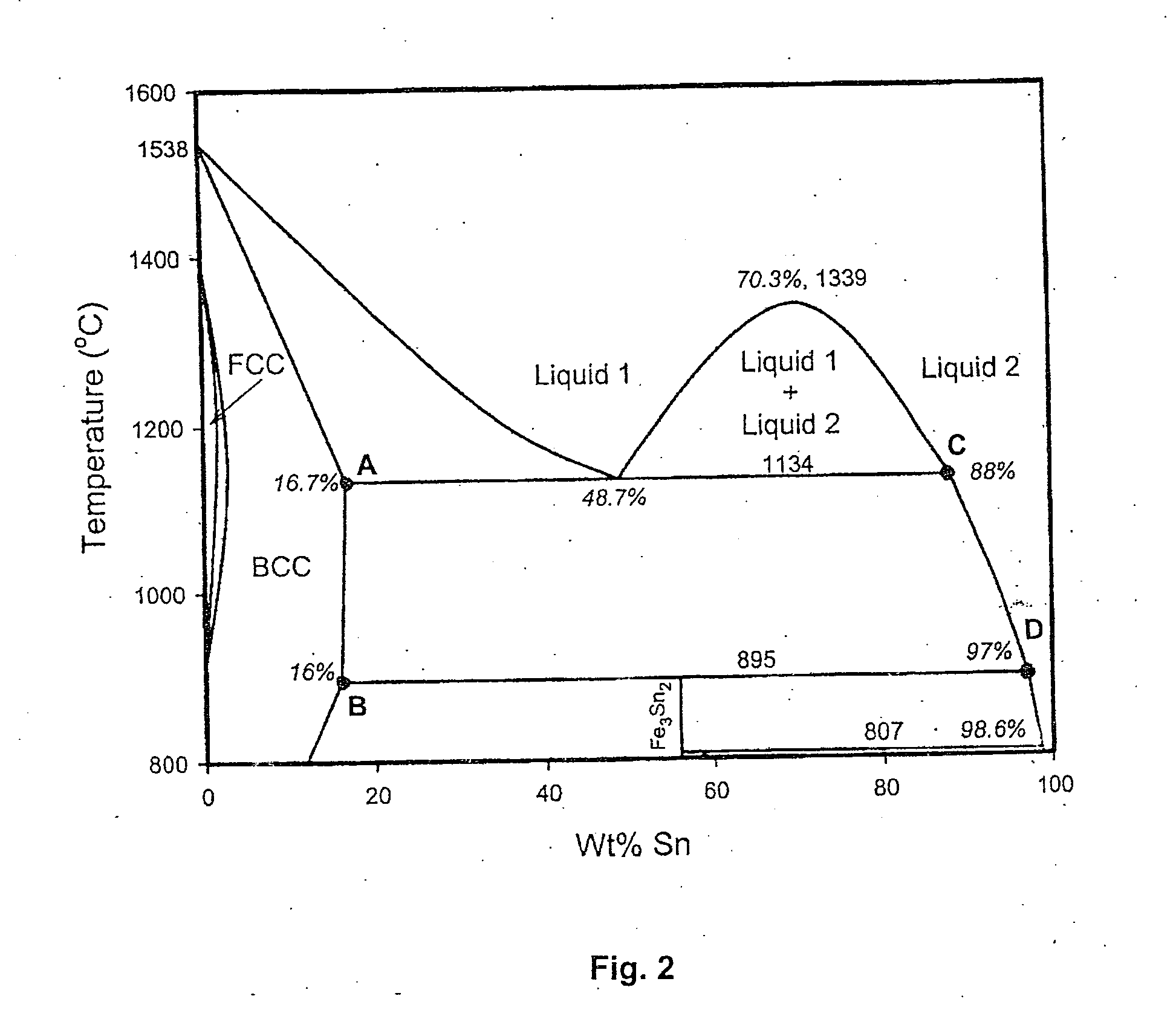
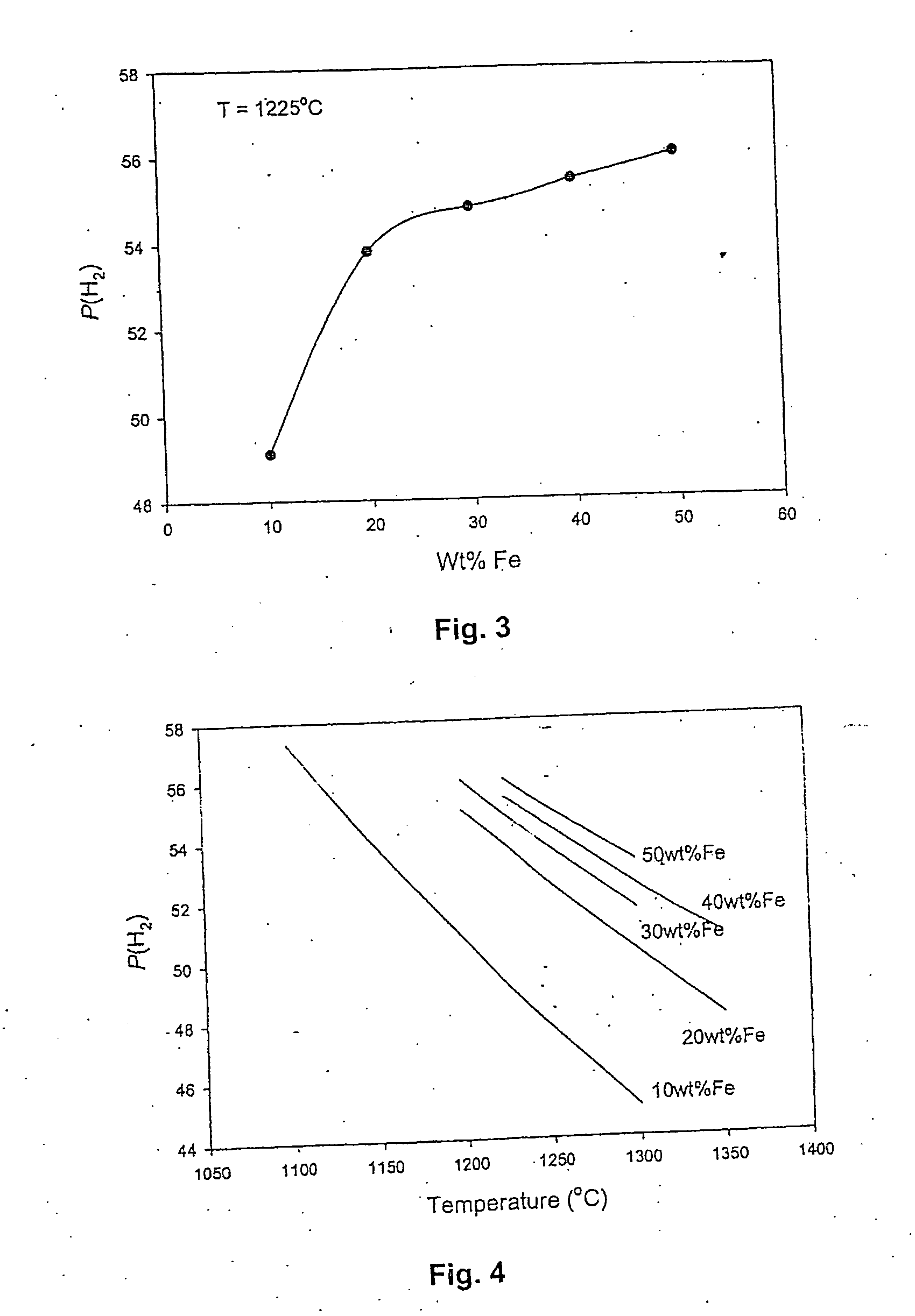
Method for the production of hydrogen-containing gaseous mixtures
a technology of hydrogen-containing gaseous mixture and gaseous mixture, which is applied in the direction of combustible gas production, combined combustion mitigation, molten salt/metal gasification, etc., can solve the problems of 70 percent of waste to the atmosphere, 30 percent of the heat generated by burning coal is converted into electricity, and the natural gas price for consumers has increased substantially
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
In this Example 1, a synthesis gas with a target molar H2:CO ratio of about 2:1 was produced. This is the H2:CO ratio that is required to make methanol.
A reactor was fabricated using an alumina closed-ended tube (2″ ID×19″ long) placed inside a stainless steel, closed-ended three-inch diameter pipe. The open end of the pipe was sealed with a flange. A one-inch exhaust line was provided to carry the synthesis gas from the reactor through a port on the flange. After exiting the reactor, the synthesis gas proceeded through a water-chilled condenser. Immediately after exiting the condenser, the synthesis gas was sampled using TEDLAR bags. The synthesis gas proceeded through an ice-chilled condenser, where it was scrubbed of particulates. Finally, the synthesis gas entered a floating drum, where the volumetric flow was measured.
A ½″OD stainless steel lance was inserted through a second port in the flange and extended into the reactor. A flow of either steam or inert gas could be inj...
example 2
10 grams of coal were injected with steam into a molten tin-iron bath (50 wt. % tin and 50 wt. % iron) having a temperature of 1200° C. The steam flow rate was 1.5 lbs / hr (14 l / min at standard conditions, 70 l / min at tubing) and the coal was injected in 2 gram charges, 5 charges total (10 grams) at a rate of 1 charge every 1-2 minutes.
After injecting steam for 7 minutes into the molten metal (to stabilize H2 production), coal was fed into the reactor in 2-gram charges. The coal and steam were then injected into the molten metal through the lance. A new charge of coal was fed into the line every 1.5 to 2 minutes. In this manner, from the initial charge, 10 grams of coal were fed into the reactor over 6.5 minutes. Gas chromatograph samples were taken of the exhaust gas flow 10 to 30 seconds after each charge was injected. On average, a gas flow of 6.7 liters / min was obtained. Previously, a blank run of 1.5 lb / hr of steam by itself through the process created 2.2 liters / min of hydro...
example 3
The following Example 3 is an evaluation of a process for making syngas suitable for methanol synthesis that was performed using METSIM, a computer program for complex chemical, metallurgical and environmental processes available from Proware, Tucson, Ariz.
The carbonaceous material was coal from Ohio (Ohio #6, Carroll County, Ohio) with ASTM rank hvBb. The analysis of the coal is summarized in Table 7.
TABLE 7Analysis of Ohio #6 CoalProximate AnalysisMoisture5.25(%, As Received)Volatile Matter37.19Fixed Carbon48.19Ash9.37Total100.00Heating Value12,388(Btu / lb As Received)Ultimate AnalysisCarbon71.95(%, Dry)Hydrogen5.10Oxygen7.77Nitrogen1.43Sulfur3.86ChlorineNot reportedFluorineNot reportedPhosphorousNot reportedAsh9.89Total100.00Sulfur FormsPyritic2.26(%, Dry)Sulfate0.12Organic1.48Total3.86
FIG. 9 illustrates the material balance that can be achieved using iron as the reactive metal in the synthesis gas reactor at a temperature varying from 1200° C. to 1300° C. Specifically, the c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ratio | aaaaa | aaaaa |
| Molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com