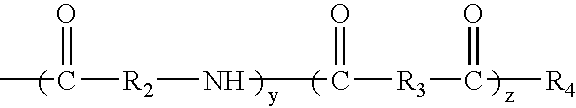
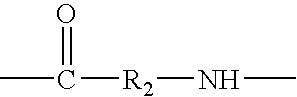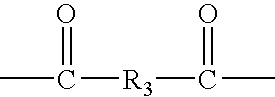Hyperbranched polymeric micelles for encapsulation and delivery of hydrophobic molecules
a technology of hydrophobic molecules and micelles, which is applied in the field of new hyperbranched colloidal polymers with micellar properties, can solve the problems of serious toxicity problems, inability to maintain long-term control over the release of drugs within the micellar microcontainer, temperature- and concentration-dependent micelles formation, etc., and achieve optimal therapeutic effects and optimal efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1-3
Example 1
Mucic Acid Propyl Ester
[0075] To a neat mixture of mucic acid (4.2 g, 20 mmol) and propionyl chloride (18 ml, 200 mmol) was added ZnCl2 (0.28 g, 2.0 mmol). The reaction mixture was heated at reflux temperature for three hours. After cooling, diethyl ether (20 ml) was added to the reaction mixture and the solution poured onto ice chips (approximately 100 g) with stirring. Additional diethyl ether (80 ml) was added to the mixture and stirring continued for 30 minutes more. The ether portion was separated, washed with water to a neutral pH, dried over anhydrous Na2SO4 and evaporated to dryness. The crude product was purified by recrystallization from a cosolvent system of diethyl ether and methylene chloride, collected by vacuum filtration, washed by ice cold methylene chloride and dried at 105° C. (12 hours) to constant weight. A white solid having a Tm of 196° C. was obtained at a 56% yield.
example 2
Mucic Acid Hexyl Ester
[0076] Mucic acid hexyl ester was prepared as in Example 1, substituting caproyl chloride for propionyl chloride. A white solid having a Tm of 171° C. was obtained at a yield of 68%.
example 3
Mucic Acid Lauryl Ester
[0077] Mucic acid lauryl ester was prepared as in Example 1, substituting lauryl chloride for propionyl chloride. A white solid having a Tm of 145° C. was obtained at a yield of 65%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com