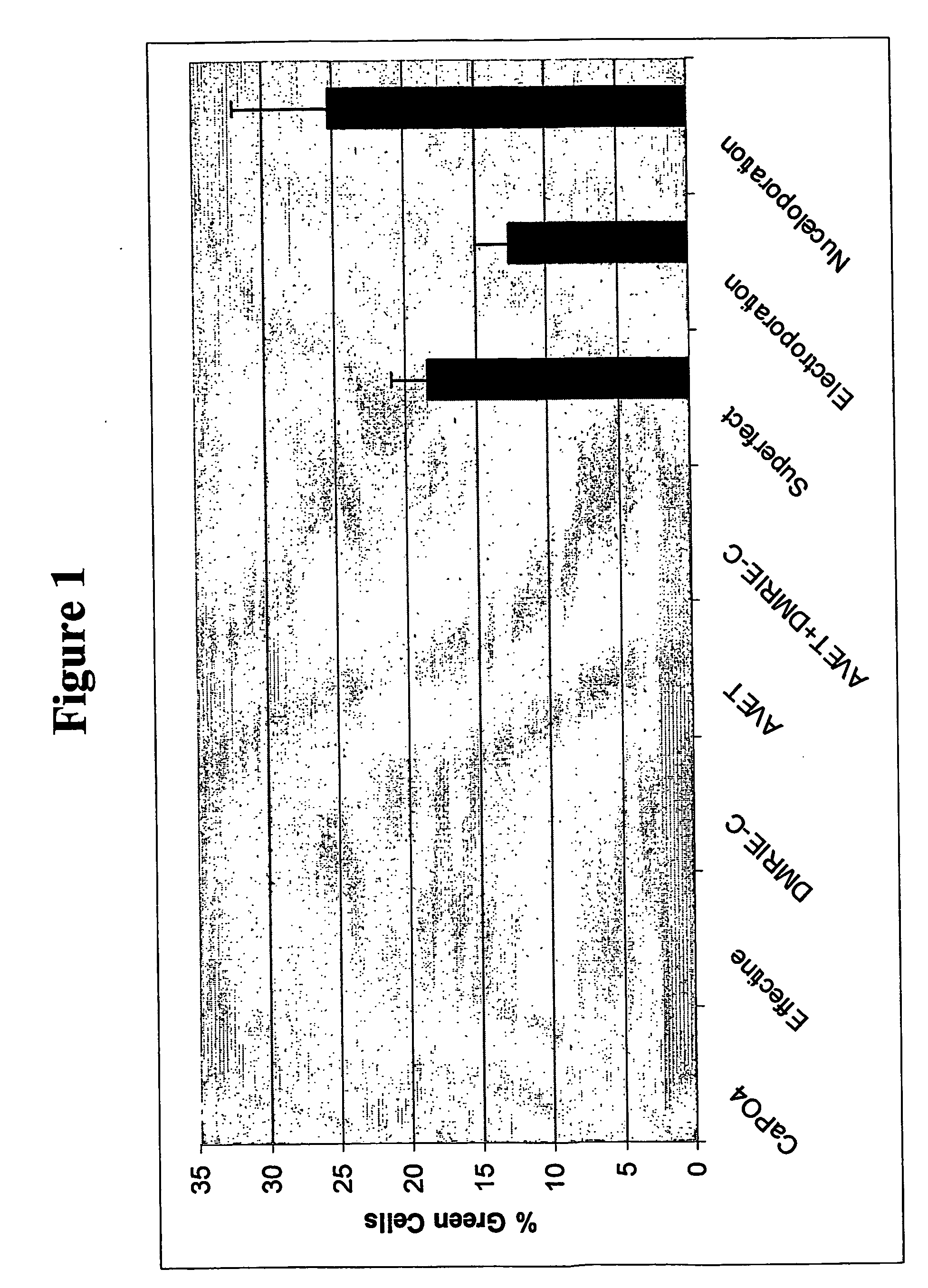
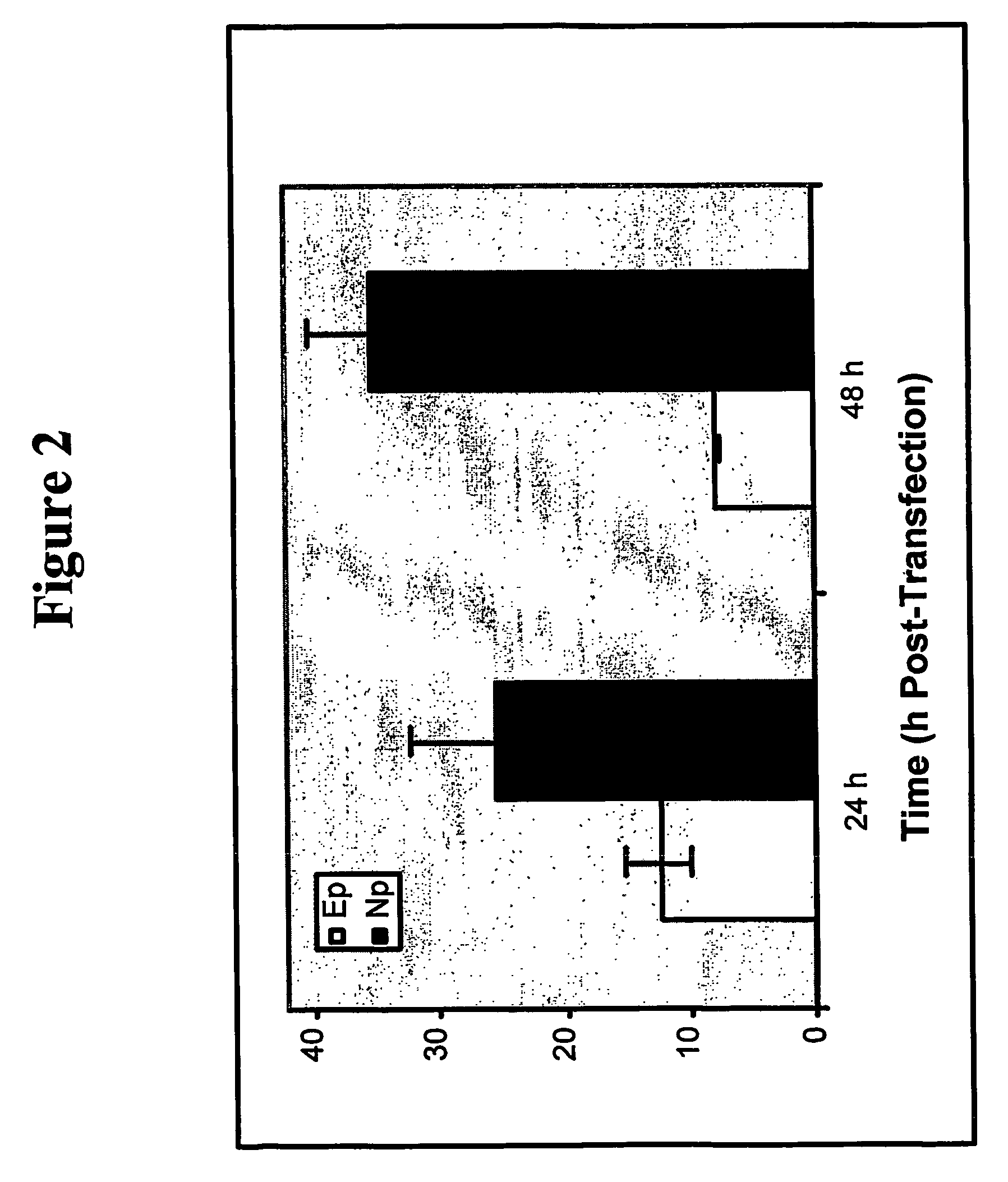
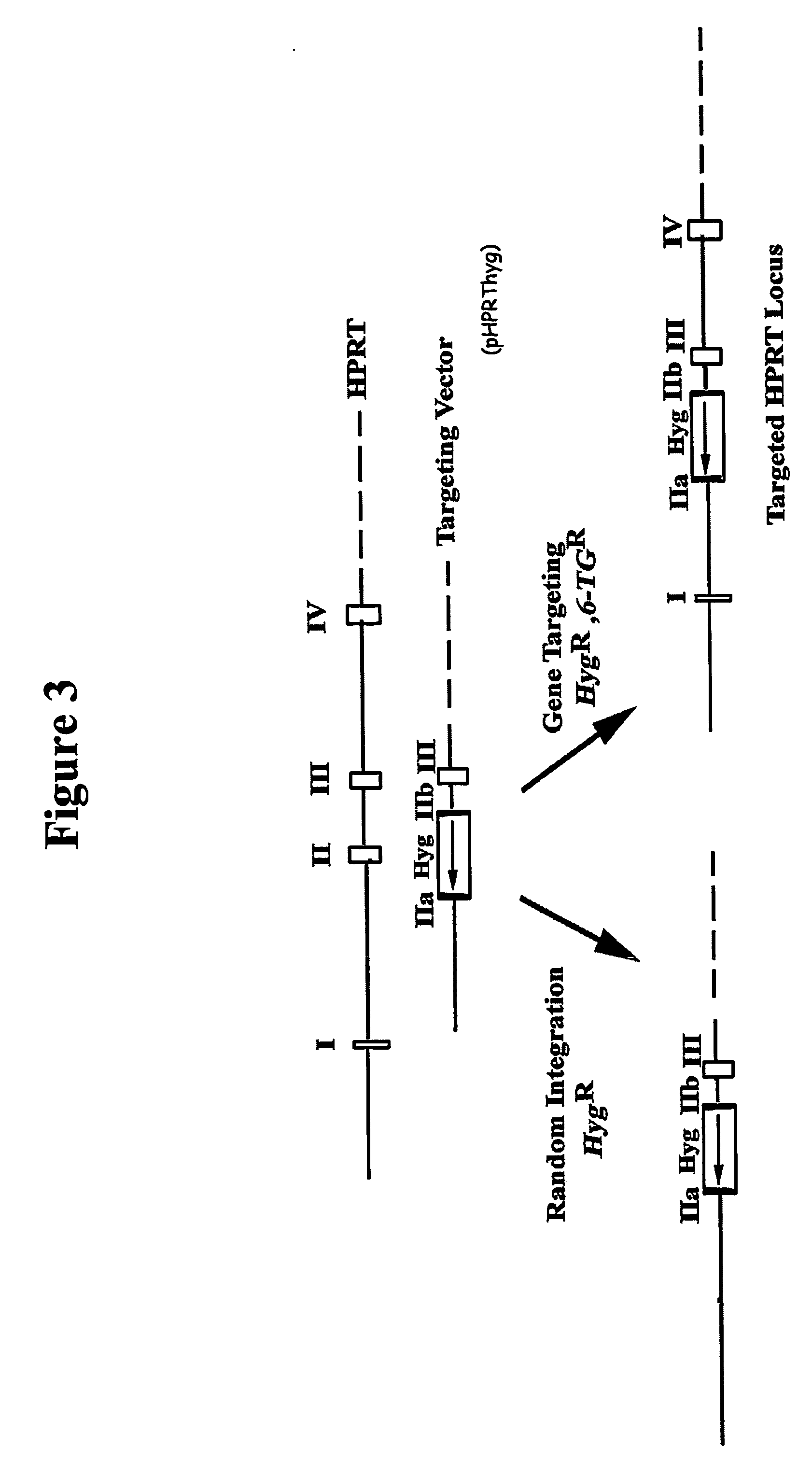
Homologous recombination in multipotent adult progenitor cells
a multi-potent adult progenitor cell and homologous recombination technology, applied in the direction of active genetic ingredients, artificial cell constructs, gene material ingredients, etc., can solve the problems of limited cell-based therapy use, inability to provide cells for repair of other damaged tissues, and cells of the hematopoietic lineage, so as to increase or decrease the production of gene products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation, Culture and Characterization of Mouse MAPCs
[0169] Mouse MAPCs (mMAPCs) were isolated, cultured and characterized essentially as described in Furcht et al. (PCT / US00 / 21387). All tissues were obtained according to guidelines from the University of Minnesota IACUC. Briefly, bone marrow mononuclear cells were obtained using Ficoll-Plaque density gradient centrifugation of bone marrow tissue from 5-6 week old ROSA26, C57 / BL6, or FANCC− / − mice.
[0170] Alternatively, muscle and brain tissue was obtained from 3-129 day old ROSA26 or C57 / BL6 mice. Muscles from the proximal parts of fore and hind limbs were excised and thoroughly minced. The tissue was treated with 0.2% collagenase (Sigma Chemical Co, St Louis, Mo.) for 1 hour at 37° C., followed by 0.1% trypsin (Invitrogen, Grand Island, N.Y.) for 45 minutes. Cells were then triturated vigorously and passed through a 70-um filter. Cell suspensions were collected and centrifuged for 10 minutes at 1600 rpm. Brain tissue was dissect...
example 2
Isolation, Culture and Characterization of Human MAPCs
[0173] Bone marrow tissue was obtained from healthy volunteer donors (age 2-50 years) after informed consent using guidelines from the University of Minnesota Committee on the use of Human Subject in Research. BMMNCs were obtained by Ficoll-Plaque density gradient centrifugation and depleted of CD45+ and glycophorin-A+ cells using micromagnetic beads (Miltenyii Biotec, Sunnyvale, Calif.).
[0174] Approximately 5×103 CD45− / GlyA− cells were diluted in 200 μL expansion medium [58% DMEM-LG, 40% MCDB-201 (Sigma Chemical Co, St Louis, Mo.), supplemented with 1× insulin-transferrin-selenium (ITS), 1× linoleic-acid bovine serum albumin (LA-BSA), 10−8 M Dexamethasone, 10−4 M ascorbic acid 2-phosphate (all from Sigma), 100 U penicillin and 1,000 U streptomycin (Gibco) and 0-10% fetal calf serum (FCS) (Hyclone Laboratories, Logan, Utah) with 10 ng / ml of EGF (Sigma) and 10 ng / ml PDGF-BB (R&D Systems, Minneapolis, Minn.)] and plated in wells ...
example 3
Differentiation of MAPCs into Multiple Cell Types
[0176] The differentiation ability of mouse, rat and human MAPCs was tested by adding differentiation factors (cytokines) that have previously been determined to be involved in the differentiation of ES cells into mesoderm, neuroectoderm, and endoderm. Differentiation required that cells were replated at 1-2×104 cells / cm2 in serum free medium, without EGF, PDGF-BB and LIF, but with lineage specific cytokines. Differentiation was determined by RT-PCR, functional studies, and immunohistology for tissue specific markers. The tissue specific markers used were: [0177] (a) slow twitch myosin and MyoD for muscle, [0178] (b) von-Willebrand factor (vWF) and Tek for endothelium, [0179] (c) NF200 and MAP2 for neuroectoderm, and [0180] (d) cytokeratin-18 and albumin for endoderm
[0181] The description below relates to differentiation of bone marrow-derived MAPCs. However, mMAPCs derived from muscle or brain were also tested, and could be induced...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com