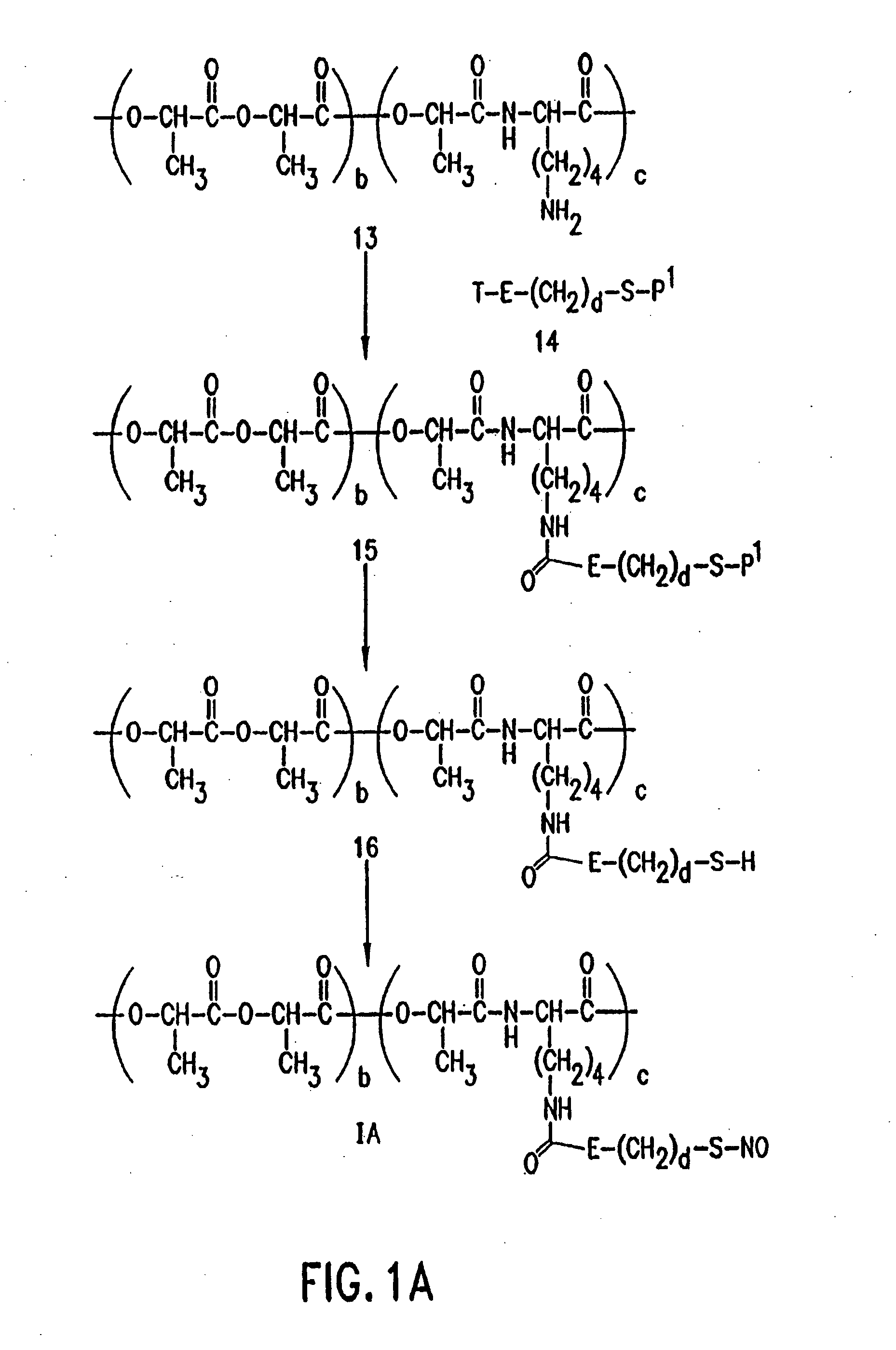
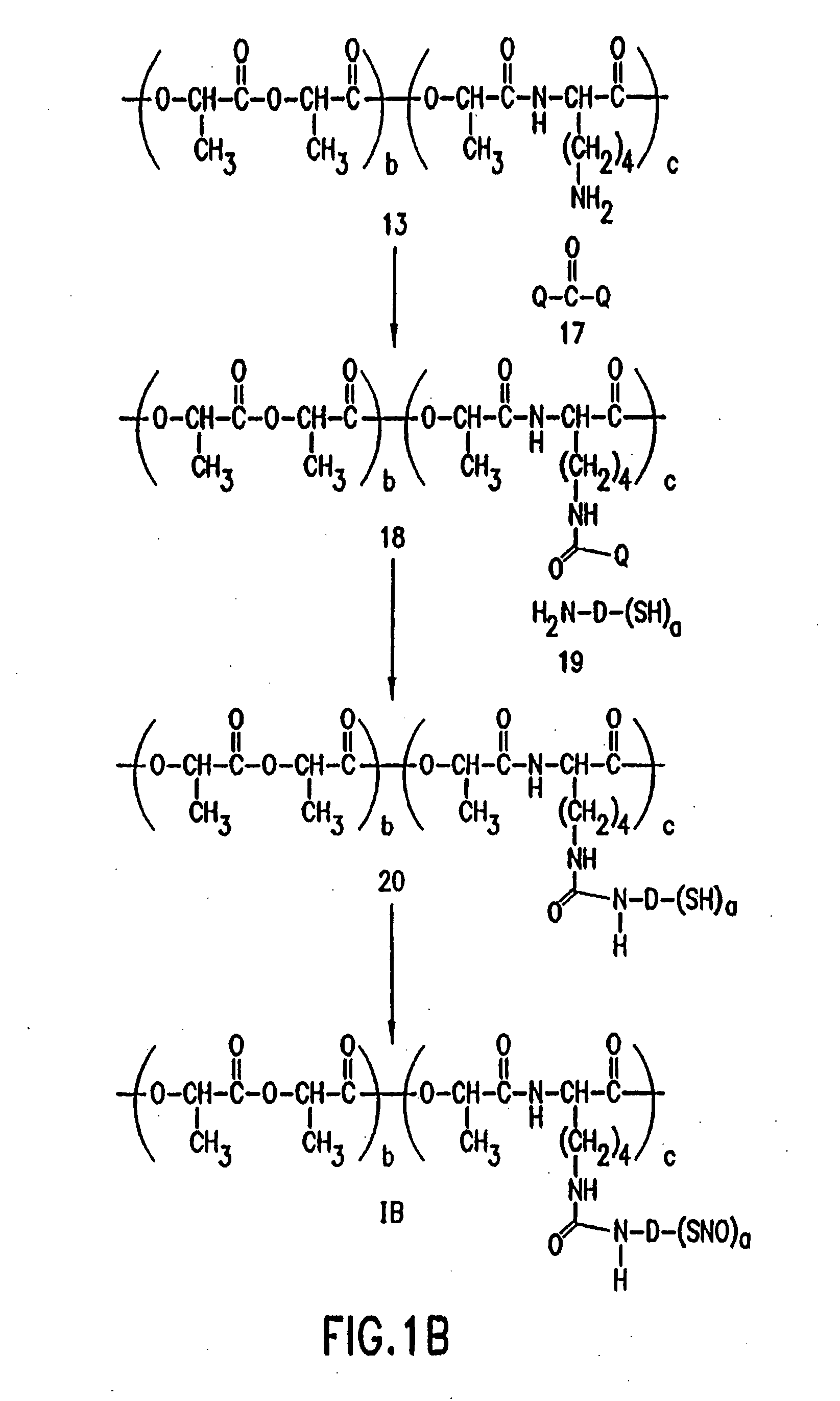
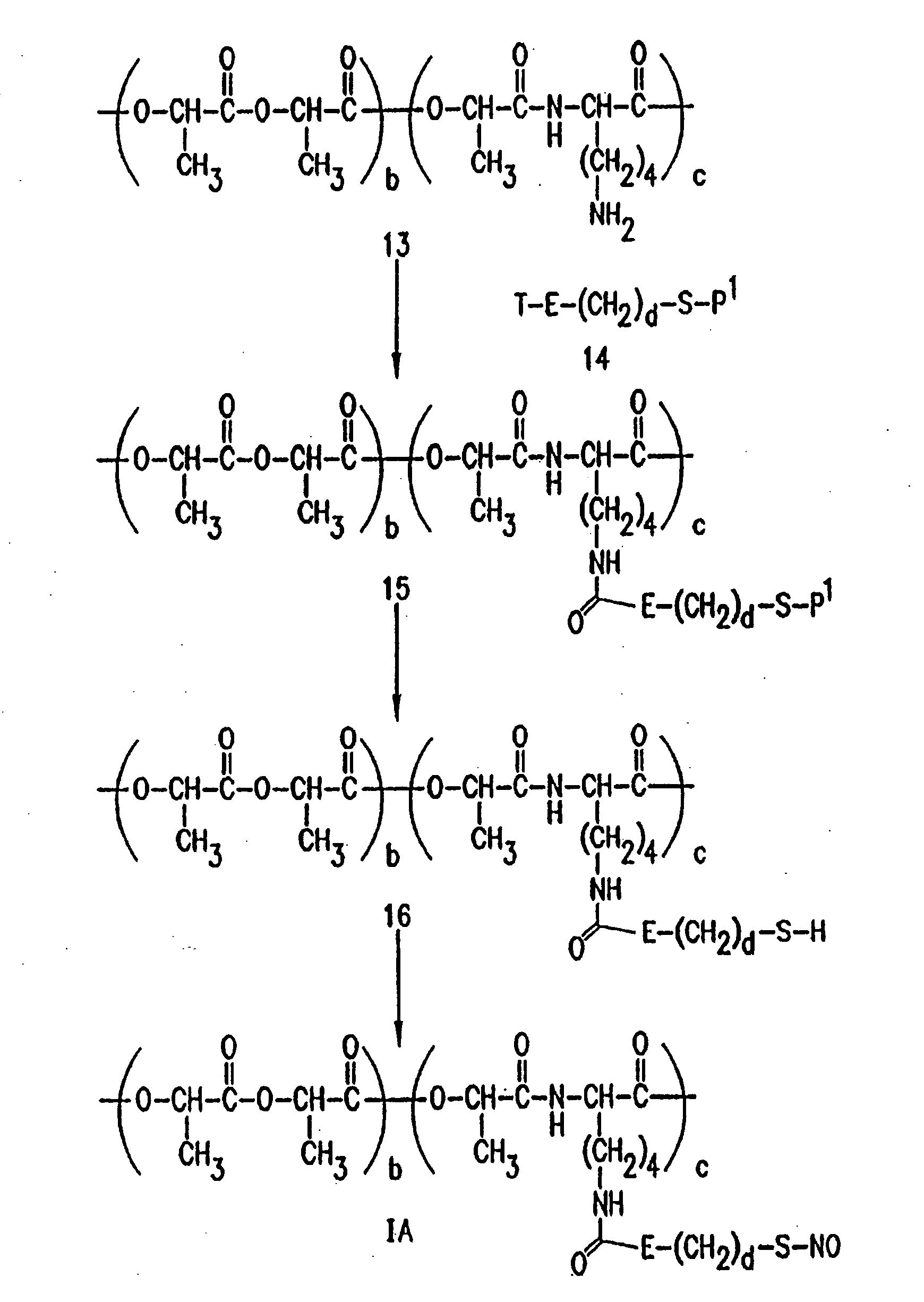Use of nitric oxide adducts
a technology of nitric oxide and adducts, which is applied in the direction of powder delivery, packaged goods type, peptide/protein ingredients, etc., can solve the problems of limited clinical usefulness of such devices, frequent exposure of blood to artificial surfaces, serious thromboembolic complications, etc., and achieve the effect of modulating the effects of vascular injury and reducing intimal proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
NO Adducts Make Artificial Surfaces Less Thrombogenic
[0141] One of the best ways to demonstrate that an artificial surface exposed to blood has been made less thrombogenic is to measure or quantitate the number of blood platelets that collect on that surface. This method requires the removal of platelets from an animal or human subject. The platelets are labeled with a radioactive material such as Indium111, which emits gamma rays, detectable by a gamma counter placed 3 to 6 inches away from the source of radioactive platelets. The labeled platelets are either reinjected into the animal or human in vivo, or contacted with the artificial surface in vivo. Platelets will adhere to artificial surfaces or acutely damaged arterial surfaces. Thus, the number of normal platelets and radioactive platelets which stick to the surface is an indication of the thrombogenicity of the surface.
[0142] The inventors have used this methodology in experiments to demonstrate that nitric oxide adducts ...
example 2
Na Nitroprusside Coated Damaged Arterial Surfaces are Less Thrombogenic
[0152] The following experiments demonstrate that nitric oxide-donating compounds, such as sodium nitroprusside and S-nitroso-BSA, can be applied directly to damaged arterial or venous surfaces (blood vessels) to inhibit platelet deposition and thrombus formation.
[0153] The inventors developed an animal model which allows them to mimic a patient with narrowing of the coronary or other arteries and arterial damage caused by atherosclerosis or after angioplasty, atherectomy or other procedure. The model uses anesthetized dogs with open chest and exposed heart. Briefly, an electromagnetic flow probe is placed on the coronary artery to continuously measure blood flow through the artery. Then the arterial wall is damaged (intima and media) by clamping the artery several times with a surgical clamp. In the area of arterial damage, a plastic encircling cylinder is placed around the outside of the coronary artery to p...
example 3
pS-NO-BSA Treats Vascular Injury
[0164] Materials: Sulfanilamide and N-(1-naphthyl) ethylenediamine dihydrochloride were purchased from Aldrich Chemical Co., Milwaukee, Wis. Sodium bicarbonate, sodium chloride, sodium phosphate, sodium nitrite, potassium phosphate-monobasic, 40% formaldehyde solution and sucrose were purchased from Fischer Scientific, Fairlawn, N.J. Sephadex G25 was purchased from Pharmacia, Piscataway, N.J., IODO-BEADS were purchased from Pierce, Rockford, Ill. and Na[125I] from New England Nuclear, Boston, Mass. [111In] oxine was purchased from Amersham, Arlington Heights, Ill. Monoclonal mouse anti-proliferating cell nuclear antigen was purchased from Dako A / S, Denmark. All other chemicals were purchased from Sigma Chemical Co., St. Louis, Mo.
[0165] Citrate-phosphate-dextrose anticoagulant solution (CPD) contained 10 mM citric acid, 90 mM trisodium citrate, 15 mM NaH2PO4H2O, and 142 mM dextrose, pH 7.35. Tris-buffered saline consisted of 10 mM tris[hydroxymethy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| internal diameter | aaaaa | aaaaa |
| internal diameter | aaaaa | aaaaa |
| internal diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com