Biodegradable polyurethane and polyurethane ureas
a technology which is applied in the field of biodegradable polyurethane and polyurethane ureas, can solve the problems of rapid loss of mechanical properties, difficulty in processing, and many currently available degradable polymers that do not meet all the requirements to be used in such applications, and achieve the effect of rapid prototyping
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 12TM4 (65% Hard Segment, 35% PCL DIOL 400).
[0082] Materials: The PCL diol (molecular weight 402.1) from ERA Polymer Pty was dried at 90° C. for 4 hours under vacuum (0.1 torr). Ethylene glycol (Aldrich) was degassed at 90° C. under vacuum (0.1 torr) for three hours and HDI (Aldrich) was used as received. A polyurethane composition based on a mixture of PCL diol, EG and HDI was prepared by a one-step bulk polymerisation procedure. Stannous octoate (Aldrich) was kept moisture-free and used as received.
[0083] A mixture of PCL (25.000 g) and EG (9.696 g) and stannous octoate (0.0714 g) was placed in a 100 ml predried polypropylene beaker, covered with aluminium foil and heated to 70° C. under nitrogen in a laboratory oven. HDI (36.732 g) was weighed in a separate wet-tared predried polypropylene beaker and added to the PCL / EG / stannous octoate beaker and stirred manually until gelation occurred (90 seconds), at which time the viscous mixture was poured onto a Teflon coat...
example 1a
Post-Synthesis Processing
[0086] The solid polymer sheet was chopped into about 1 cm3 pieces with clean tin-snips, cooled in liquid nitrogen and ground into a powder using a cryogrinder. The polymer powder was then dried at 100° C. under vacuum overnight. The polymer was extruded on a mini-extruder equipped with a 1.7 mm die at 180° C. and 40 rpm. The polymer was taken off by a belt conveyor and cooled at ambient temperature in air without water bath. The filament was spooled and kept under nitrogen in a moisture-free environment for at least one week prior to use.
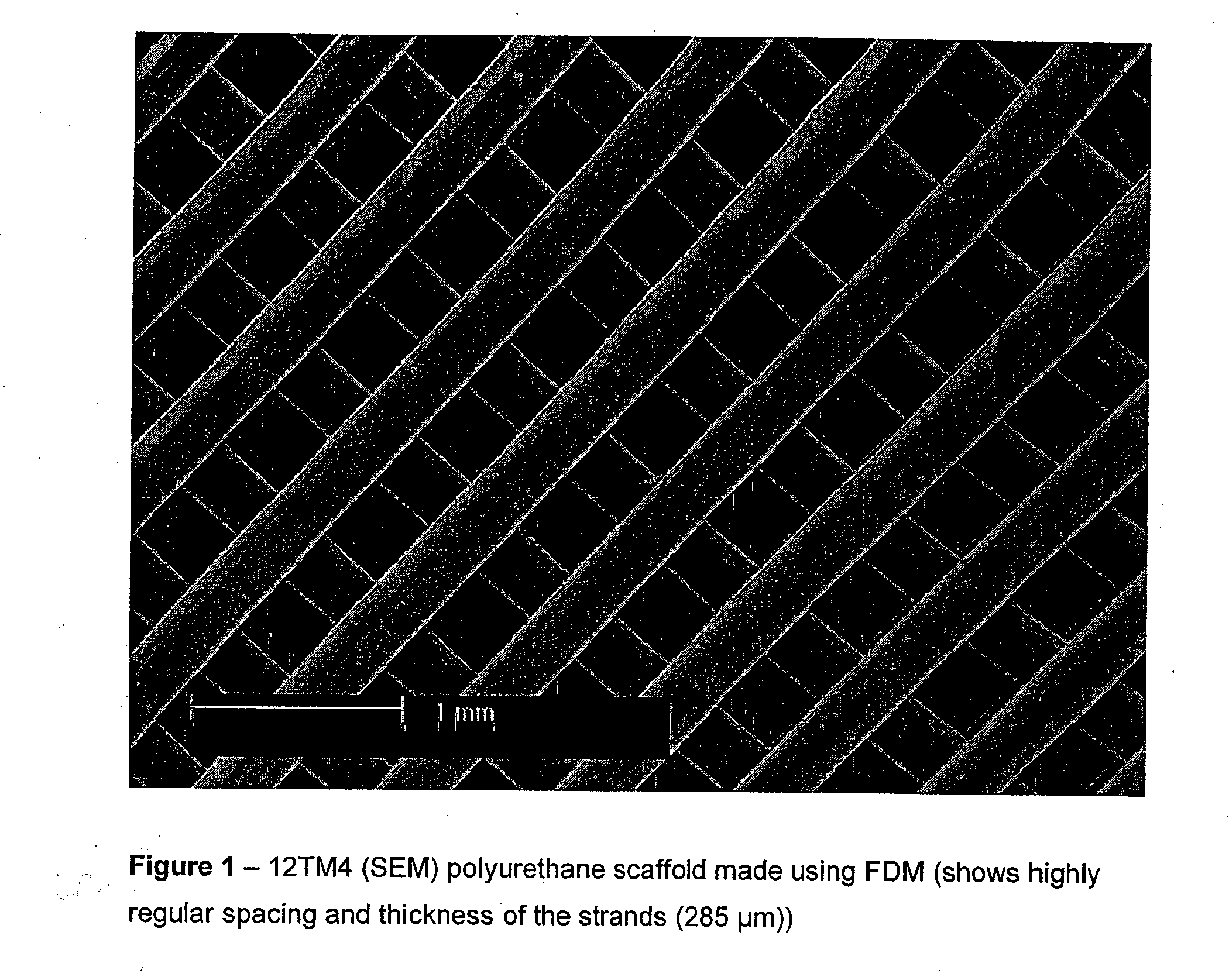
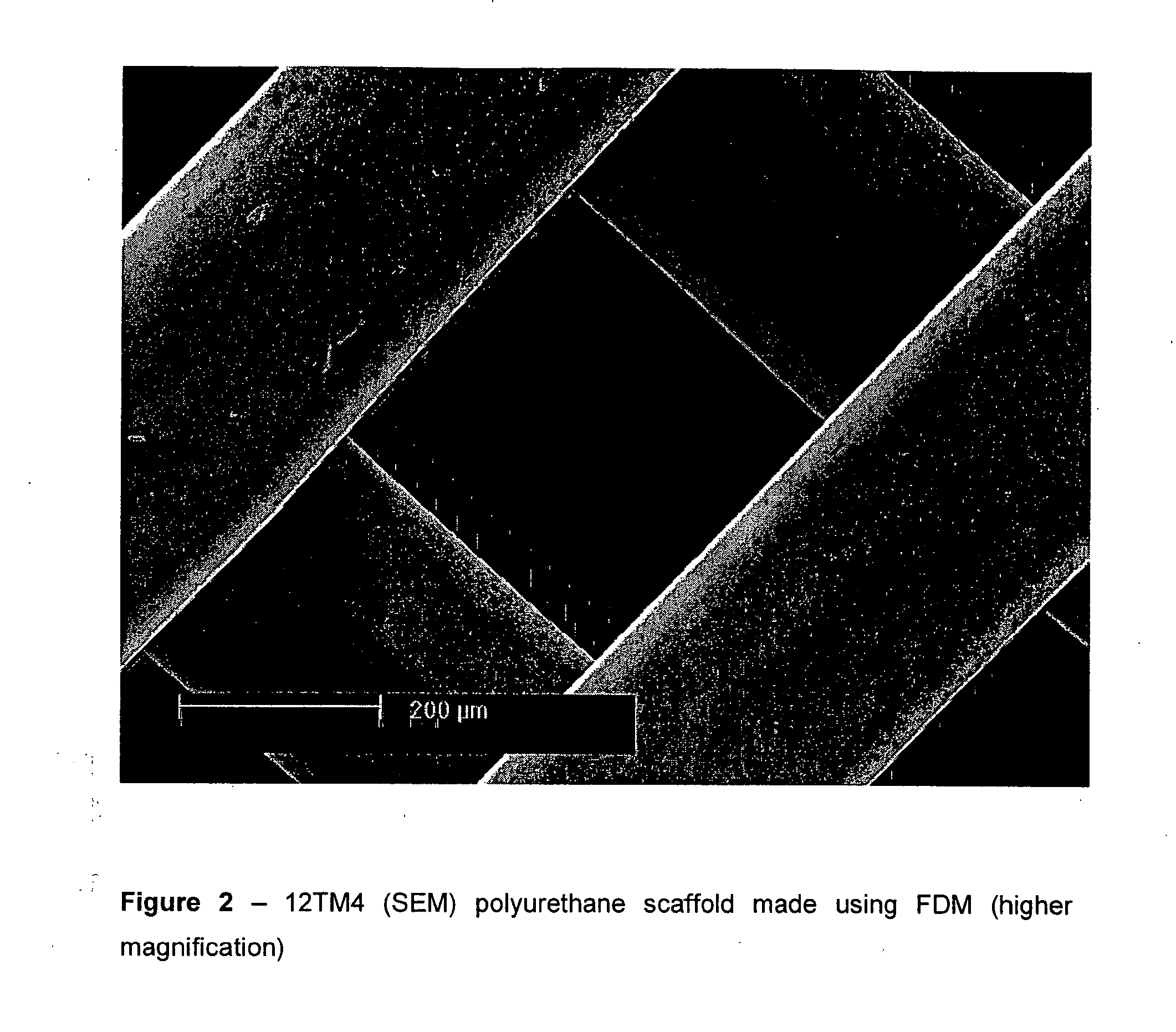
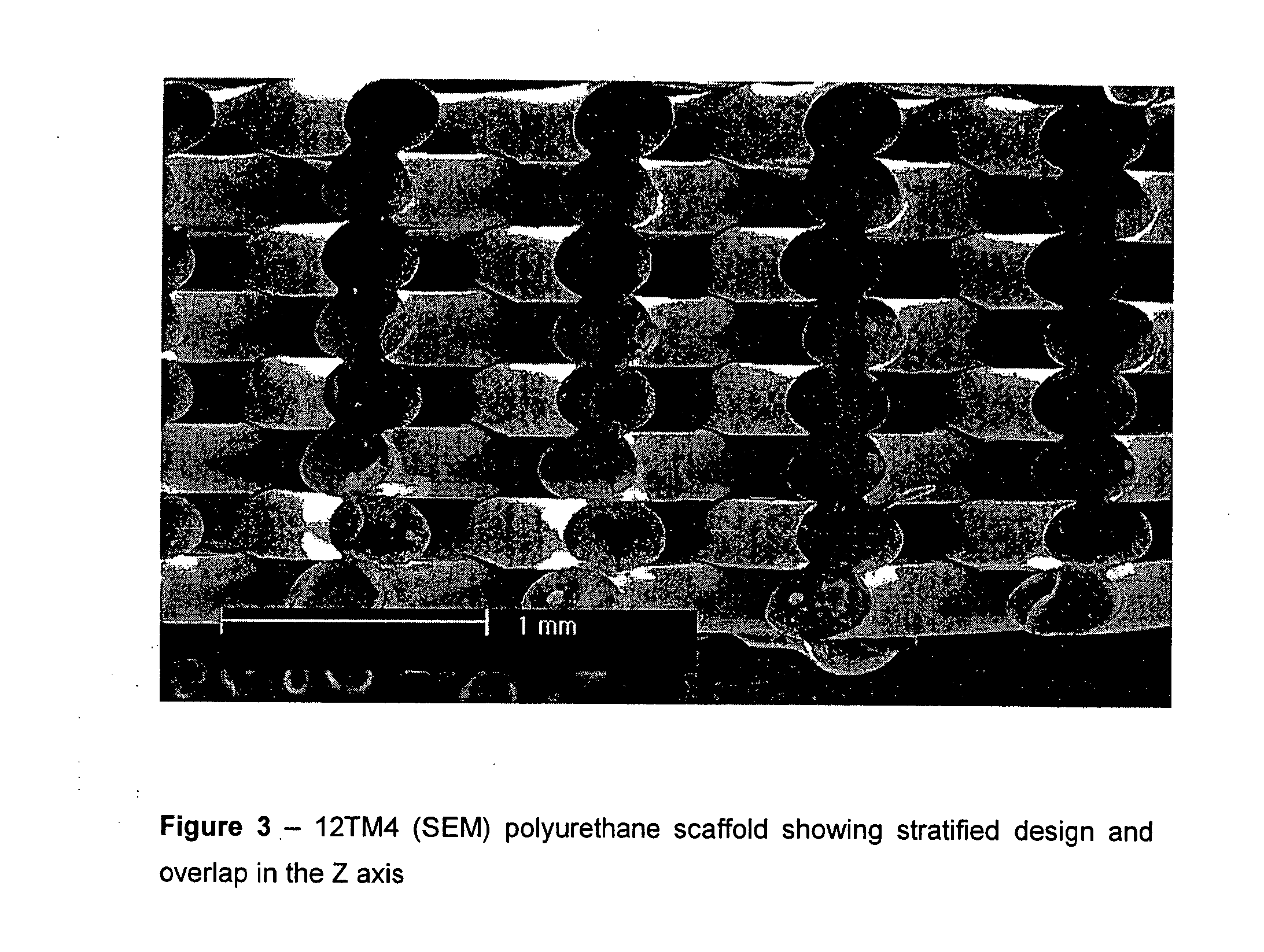
[0087] The polymer filament was fed though the FDM apparatus and a small lattice was made to show that the material was suitable for FDM. The scaffolds were characterised by light microscopy and SEM and were shown to have very good precision and weld. It has been shown to work with a number of commercially available nozzle diameters.
[0088] The operating envelope temperature inside the machine was 25° C. and the heating z...
example 2
Preparation of 12TM1 (A softer Material than Example 1, 60% HARD SEGMENT, 40% PCL DIOL 400)
[0089] Materials: The PCL diol (molecular weight 402.1) from ERA Polymer Pty was dried at 90° C. for 4 hours under vacuum (0.1 torr). Ethylene glycol (Aldrich) was degassed at 90° C. / 0.1 torr for 3 hours and HDI (Aldrich) was used as received. A polyurethane composition based on a mixture of PCL diol, EG and HDI was prepared by a one-step bulk polymerisation procedure. Stannous octoate (Aldrich) was kept moisture-free and used as received.
[0090] A mixture of PCL (40.0 g) and EG (11.663 g) and stannous octoate (0.100 g) was placed in a 100 ml predried polypropylene beaker, covered with aluminium foil and heated to 70° C. under nitrogen in a laboratory oven. HDI (48.337 g) was weighed in a separate wet-tared predried polypropylene beaker, covered and then added to the PCL / EG / stannous octoate beaker and stirred manually until gelation occurred (90 seconds). The viscous mixture was poured onto a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com