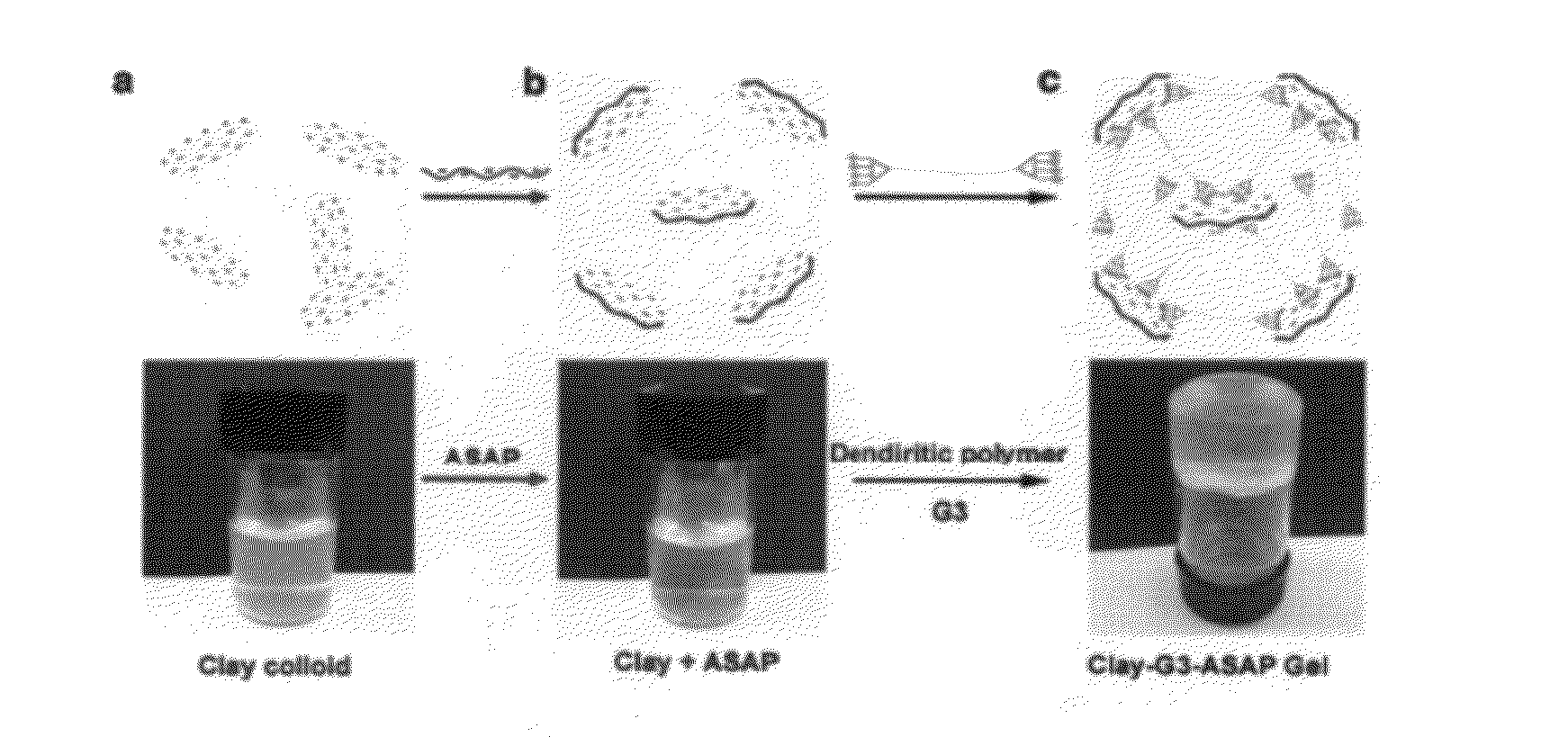
Polyionic dendrimer and hydrogel comprising same
a technology of polyionic dendrimer and hydrogel, which is applied in the direction of peptides, drug compositions, silicates, etc., can solve the problems of low strength of opaque and soft materials, no self-restoring properties, and failure to achieve high water content, easy to synthesize, and no mechanical strength decrease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0134]G1-OH, G2-OH, and G3-OH were produced in accordance with the reaction scheme shown below.
[0135]Compound 7 (G1-OH), that is a bisdendrimer having a hydroxy group as the surface, a polyester group as the branched chain, and polyethylene glycol group as the core, and its second generation compound and third generation compound, i.e., Compound 9 (G2-OH) and Compound 11 (G3-OH), were produced according to the method described in the Document (Non-patent Document 8; H. Ihre, O. L. Padilla De Jesus, J. M. J. Frechet, J. Am. Chem. Soc. 123, 5908 (2001)).
[0136]NMR and MS data of Compound 7 (G1-OH):
[0137]1H-NMR (270 MHz, CDCl3) δ:
[0138]1.09 (s, 6), 3.64 (bs, ˜1200), 4.32 (t, 4, J=5.0).
[0139]MALDI-TOF-MS: [M]+=10879.
[0140]NMR and MS data of Compound 9 (G2-OH):
[0141]1H-NMR (270 MHz, CDCl3) δ:
[0142]1.05 (s, 12), 1.29 (s, 6), 3.36 (t, 10, J=4.8),
[0143]3.64 (bs, ˜1200), 4.32 (m, 8), 4.40 (d, 4, J=11.1).
[0144]MALDI-TOF-MS: [M]+=11476.
[0145]NMR and MS data of Compound 11 (G3-OH):
[0146]1H-NMR (...
example 2
[0151]G1 was produced in accordance with the reaction scheme shown below.
(1) Production of Compound 13 (G1-NH(Boc))
[0152]1.0 g (0.09 mmol, 1 eq.) of Compound 7 (G1-OH) was dissolved in 5.0 mL of dichloromethane (CH2Cl2), added with 0.25 mL of pyridine and 0.40 g (2.0 mmol, 22 eq.) of 4-nitrophenylchloroformate, and then reacted by stirring at room temperature for 22 hours. The reaction solution was poured in diethyl ether to precipitate Compound 12, which was then purified. Compound 12 was dissolved in 6.0 mL benzene, added with 0.07 g (0.51 mmol, 5.7 eq.) of 4-(dimethylamino)pyridine (DMAP) and 0.35 g of tert-butyl 2-(2-(2-aminoethoxy)ethoxy)ethylcarbamate, and then reacted by stirring at room temperature for 12 hours. The reaction solution was poured in diethyl ether to obtain 0.8 g of Compound 13 as a white solid (yield 68%).
[0153]1H-NMR (270 MHz, CDCl3) δ:
[0154]1.20 (s, 6), 1.04 (s, 6), 1.42 (s, 36),
[0155]3.64 (bs, ˜1200), 4.19 (t, 8, J=5.0).
[0156]MALDI-TOF-MS: [M]+=11684.
(2) Pr...
example 3
[0163]G2 was produced in accordance with the reaction scheme shown below.
(1) Production of Compound 17 (G2-NH(Boc))
[0164]1.0 g (0.09 mmol, 1 eq.) of Compound 9 (G2-OH) was dissolved in 5.0 mL of dichloromethane, added with 0.25 mL of pyridine and 0.80 g (4.0 mmol, 44 eq.) of 4-nitrophenylchloroformate, and then reacted by stirring at room temperature for 12 hours. The reaction solution was poured in diethyl ether to precipitate Compound 16, which was then purified. Compound 16 was dissolved in 6.0 mL of benzene, added with 0.14 g (1.02 mmol, 11.4 eq.) of 4-(dimethylamino)pyridine (DMAP) and 0.70 g of tert-butyl 2-(2-(2-aminoethoxy)ethoxy)ethylcarbamate, and then reacted by stirring at room temperature for 12 hours. The reaction solution was poured in diethyl ether to obtain 0.71 g of Compound 17 as a white solid (yield 55%).
[0165]1H-NMR (270 MHz, CDCl3) δ:
[0166]1.17 (s, 12), 1.24 (s, 6), 1.42 (s, 72),
[0167]3.64 (bs, ˜1200), 4.16 (t, 16, J=5.0).
[0168]MALDI-TOF-MS: [M]+=12542.
(2) Prod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| transparent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com