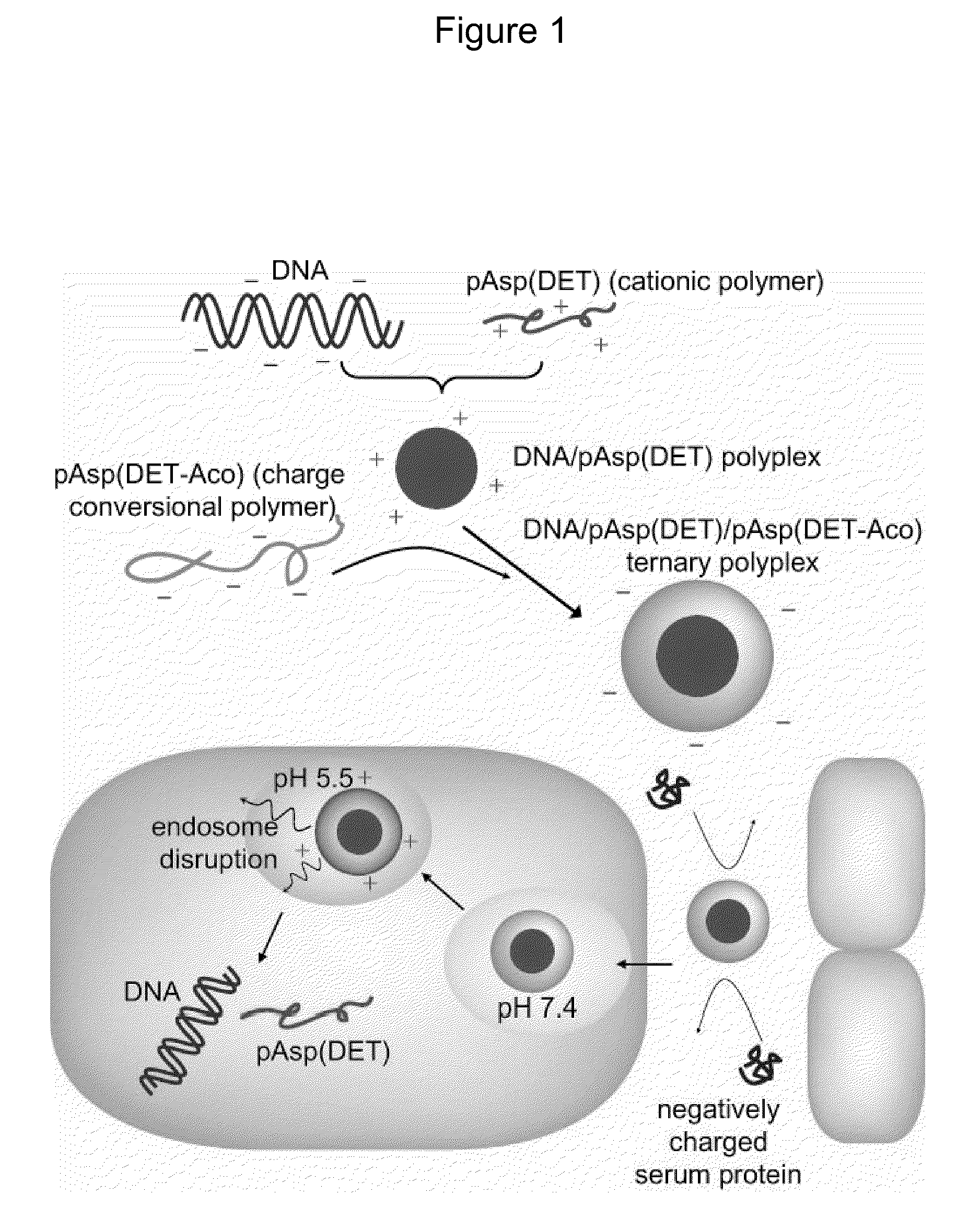
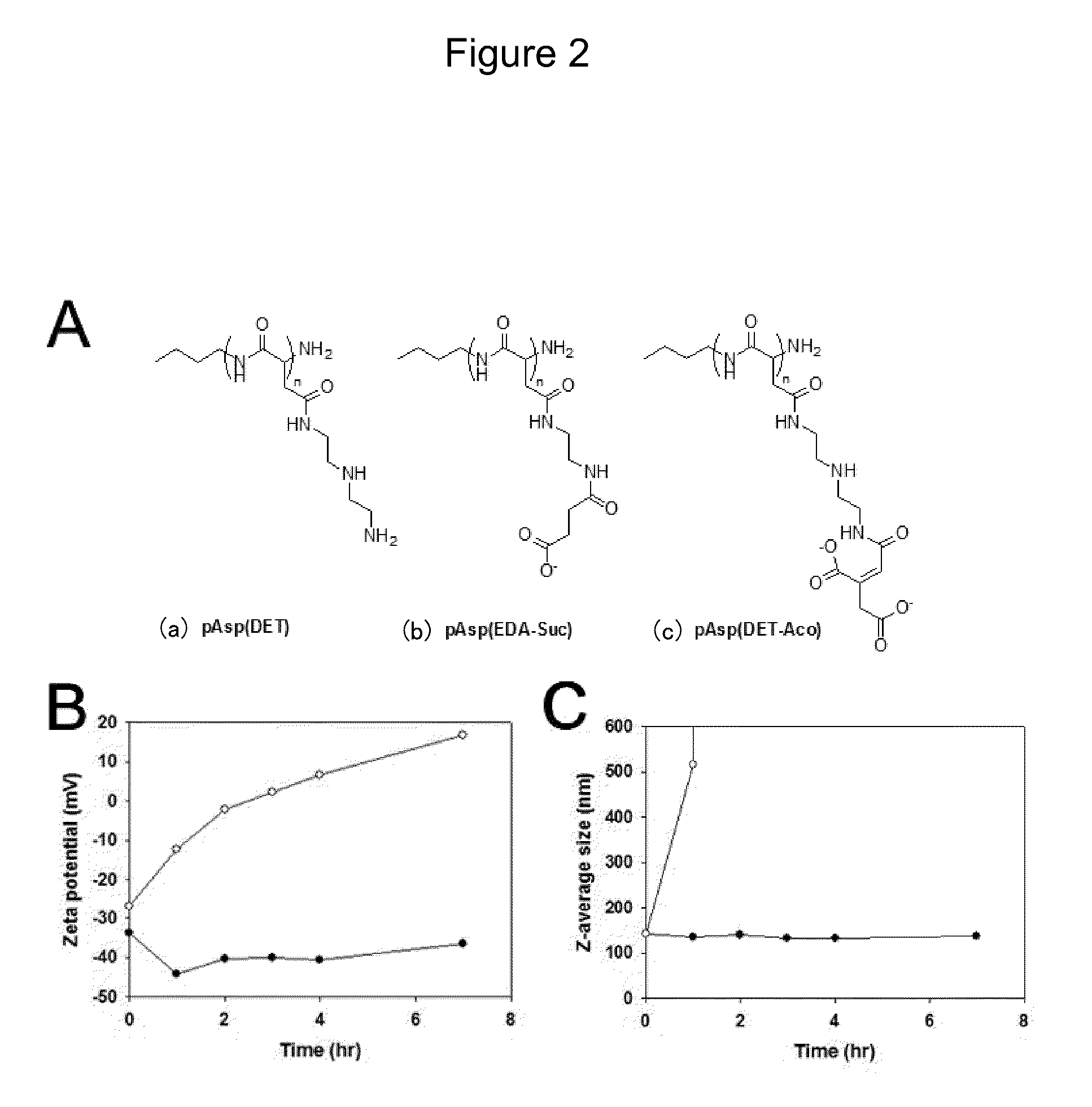
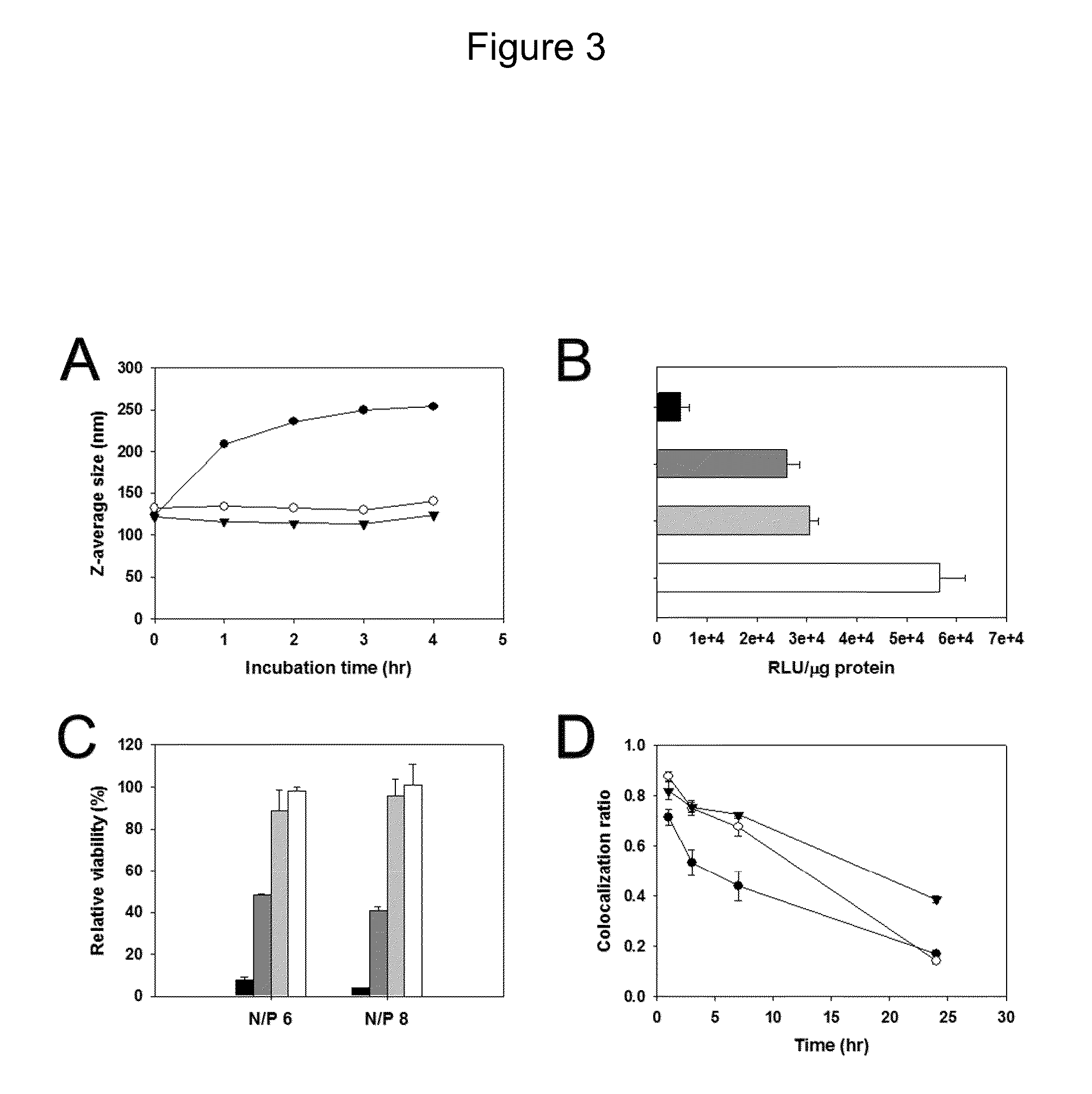
Charge conversional ternary polyplex
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0136]1. Materials
[0137]N,N-dimethylformamide (DMF) (Wako Pure Chemical Industries, Ltd, Japan), dichloromethane (DCM) (Wako, Japan), n-butylamine, ethylenediamine (1,2-diaminoethane), and diethylenetriamine (bis(2-aminoethyl)amine) (Tokyo Chemical Industry Co. Ltd, Japan) were purchased and redistilled before use. Acetic acid and hydrochloric acid were purchased and used without further purification (Wako, Japan). 1-methyl-2-pyrrolidinone (NMP), cis-aconitic anhydride, succinic anhydride and bovine serum albumin were purchased from Sigma (St. Louis, Mo.). β-benzyl-L-aspartate-N-carboxy-anhydride (BLA-NCA) was obtained from Nippon Oil and Fats Co., Ltd. (Tokyo, Japan).
[0138]2. Synthesis
[0139]2-1. Synthesis of PBLA (poly([3-benzyl-L-aspartate)) (2)
[0140]PBLA was prepared by ring-opening polymerization of BLA-NCA initiated by the terminal amino group of n-butylaminc. n-butylamine (0.0417 mmol) was dissolved in 5 mL of DMF / DCM (1:10). BLA-NCA (4.60 mmol) solution i...
example 2
Experimental Method
[0176](1) Synthesis of Poly(L-lysine) (PLL) Homopolymer
[0177]An eggplant shaped flask provided with a three-way stopcock was vacuum-dried, added with Lys(TFA)-NCA (1.01 g, 3.77 mmol) under an argon (Ar) atmosphere, and then added with DMSO (8 mL) for lysis. As an initiator, n-butylamine (5.4 mg, 73.8 μmol) was added to the monomer solution, and agitated for about 48 hours in a thermostatic bath at 35° C. After the reaction, the polymerization solution was slowly dripped in an excessive amount of thoroughly agitated diethyl ether to precipitate the polymer. Once most polymer has precipitated after about an hour of agitation, supernatant solution was removed by decantation. The precipitated polymer was dried under reduced pressure at room temperature. According to 1H NMR analysis, the chain length (degree of polymerization (DP) of lysine) was 52.
[0178]50 mg of P[lys(TFA)] (DP: 52) was dissolved in 5 ml of MeOH, added with 0.5 ml of 1N NaOH and agitated at 35° C. for...
example 3
Experimental Method
[0212](1) Synthesis of N-Succinimidyl Octadecanoate
[0213]N-succinimidyl octadecanoate was synthesized according to a known method [N. M. Howarth, W. E. Lindsell, E. Murray, P. N. Preson, Tetrahedron 61 (2005) 8875-8887]. Stearic acid (1.87 g, 6.56 mmol) and N-hydroxysuccinimide (0.76 g, 6.56 mmol) was dissolved in 80 mL of dichloromethane (DCM), and allowed to react with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (WSC) (1.25 g, 6.56 mmol) for 48 hours. Thereafter, the resultant was washed with water, extracted twice with DCM and dried with MgSO2 to obtain white powder (amount 1.4 g, yield 56%). The conversion ratio of the carboxyl group of stearic acid was 96% as calculated by 1H-NMR.
[0214](2) Synthesis of Stearoyl Group-Introduced Poly(L-Lysine)
[0215]poly(L-Lysine) (molecular weight 20,000) and DIPEA were dissolved in methanol, and allowed to react with N-succinimidyl octadecanoate dissolved in a small amount of dichloromethane at 4° C. for 24 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com