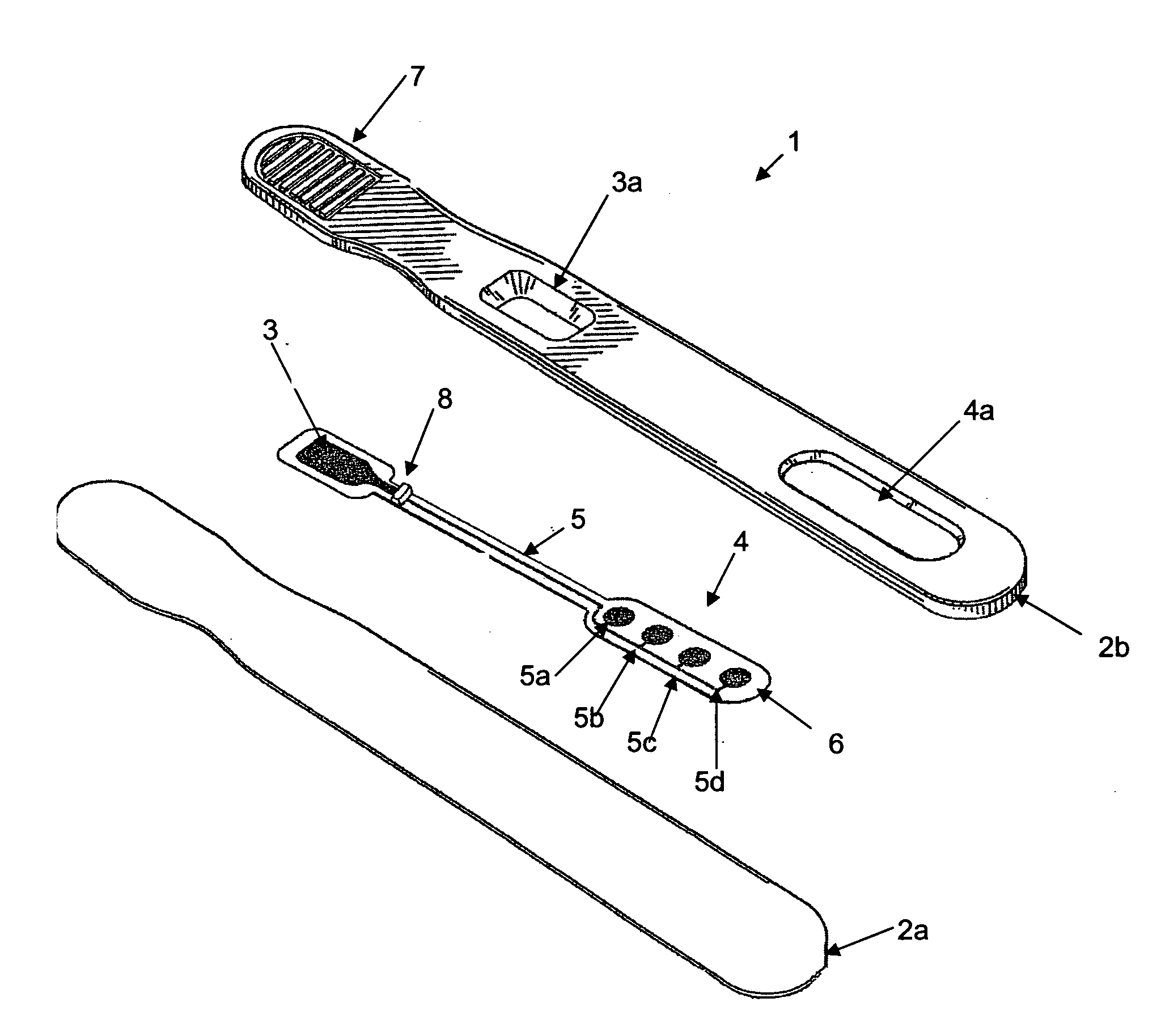
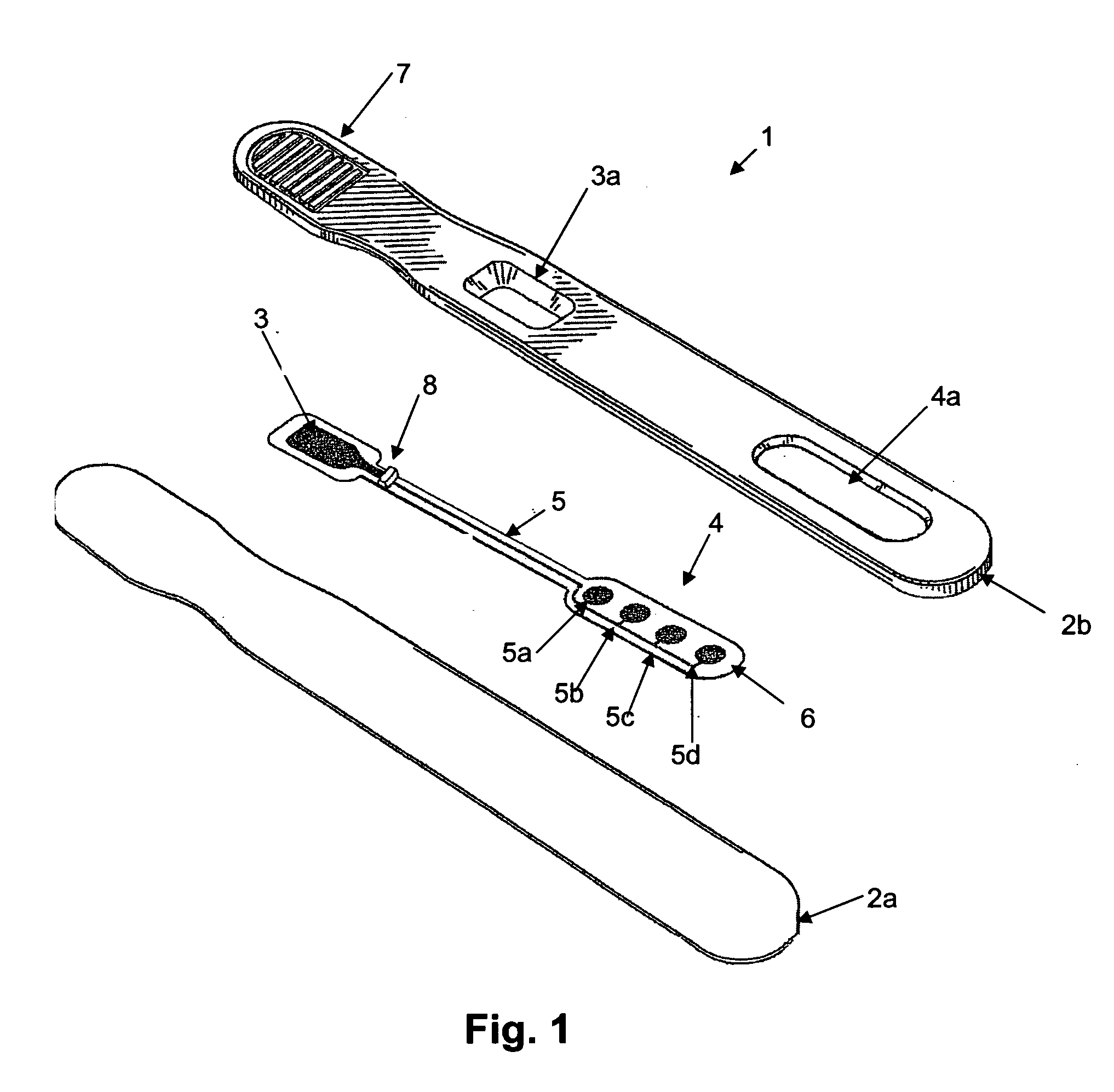
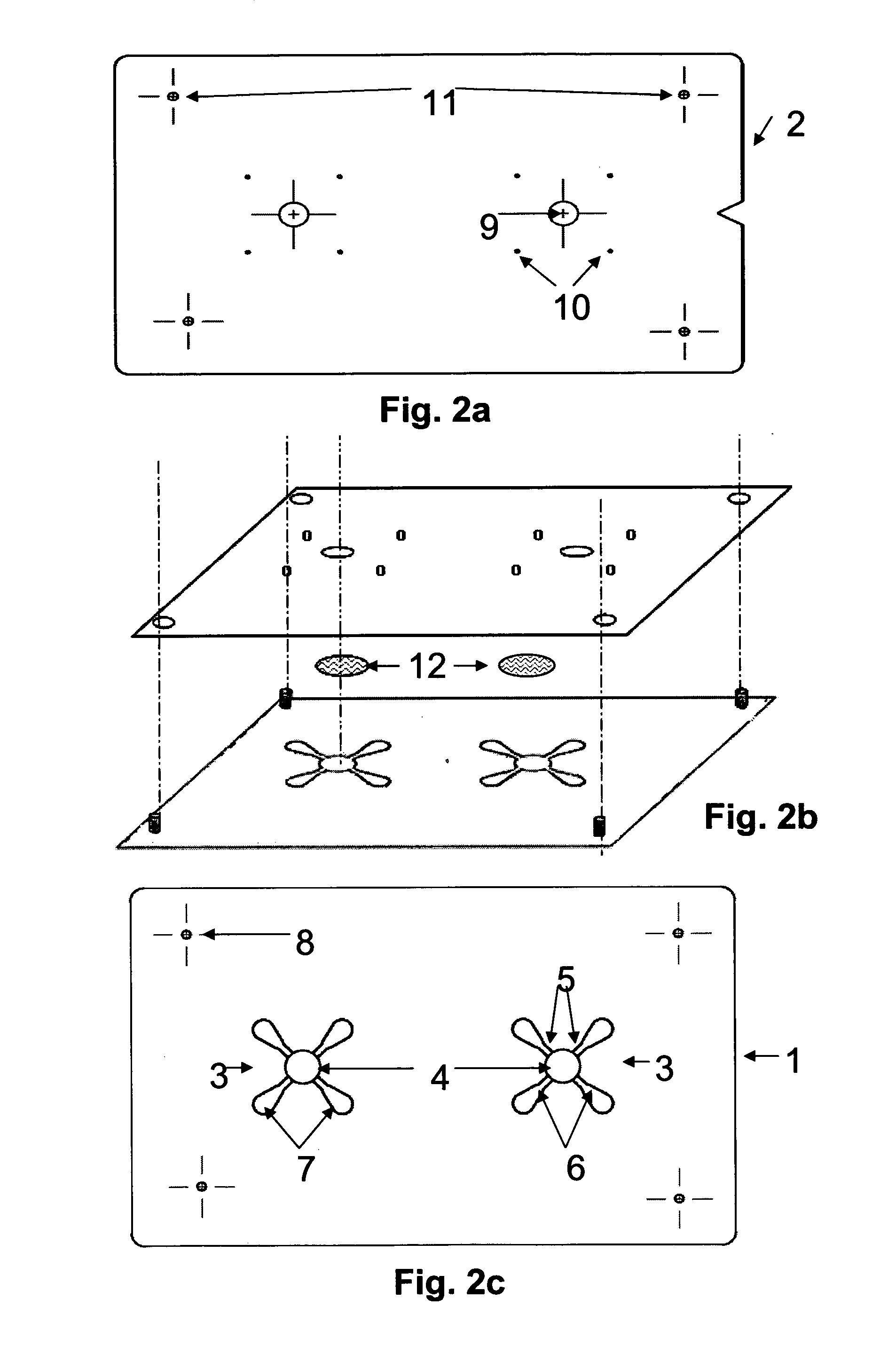
Assay device and methods
a technology of assay device and method, which is applied in the field of amphipathic polymers, can solve the problems of adverse effect on the accuracy of the device, uncontrollable flow, and inability to collect a large sample, and achieve the effects of rapid and inexpensive point of care cholesterol assay system, high efficiency and/or speed of assay, and easy quantification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0174]The assay utilizes a triple enzyme system capable of converting one molecule of cholesterol or cholesterol ester into a molecule of hydrogen peroxide (H2O2). The hydrogen peroxide generated is then used to oxidize the dye Amplex Red (non-fluorescent) to generate the highly fluorescent product Resorufin.
Stabilization of Enzymes onto Plastic
The total cholesterol assay uses the following enzymes and dye:
Cholesterol Esterase (3.1.1.13)
Cholesterol Oxidase (1.1.3.6)
Horseradish Peroxidase (1.11.1.7)
[0175]Amplex Red: 10-acetyl-3,7-dihydroxyphenoxazine
[0176]Enzymes were stabilized onto plastic using Gafquat as a stabilizing agent. The three enzymes were added to a solution of 0.01M potassium Phosphate buffer, pH 7.0. Final activity of each enzyme was measured at 200 U / ml of buffer. The solution was then diluted 1:1 with Gafquat (highly positively charged polymer) formulation and 5 ul of the resulting solution was deposited onto a plastic surface and dried at 30° ...
example 2
Total Lipid Assay
[0191]The K37 dye was dissolved in DMF to a final concentration of 1.0 mM. Next 5% w / v PEG 2000 was dissolved into the dye solution and 60 nanolitres of the resulting solution was deposited onto a plastic surface and dried by removing the solvent under vacuum for 1 hour at room temperature in the dark.
Assay Procedure—Utilising Diluted Sample
[0192]The sample to be assayed (plasma) was first diluted 1 part into 80 parts phosphate buffered saline containing 50 mM Sodium Octanoate, pH 7.4.
[0193]5 μl of diluted plasma sample was applied to the dried dye which spontaneously hydrated. The Total lipid content was measured by exciting the sample at 440 nm (10 nm bandpass) and measuring the resulting fluorescence passing through a 495 nm filter (10 nm bandpass). The total lipid content was determined empirically by reference to known standards.
Assay Procedure—Utilising Undiluted Sample
[0194]Neat biological samples are assayed by applying the sample to a borosilicate filter im...
example 3
HDL Cholesterol Assay
[0195]Nile Red was dissolved in DMF to a final concentration of 0.5 mM. Next 5% w / v PEG 2000 was dissolved into the dye solution and 60 nanolitres of the resulting solution was deposited onto a plastic surface and dried by removing the solvent under vacuum for 1 hour at room temperature in the dark.
Assay procedure—Utilising Diluted Sample
[0196]The sample to be assayed (plasma) was first diluted 1 part into 80 parts phosphate buffered saline containing 50 mM Sodium Octanoate, pH 7.4.
[0197]5 μl of diluted plasma sample was applied to the dried dye which spontaneously hydrated. The HDL cholesterol content was measured by exciting the sample at 580 nm (10 nm bandpass) and measuring the resulting fluorescence passing through a 610 nm filter (10 nm bandpass). HDL cholesterol content was calculated by use of the algorithm described in the main specification. An equivalent assay may also be performed utilising whole, undiluted blood.
Assay procedure—Utilising Undiluted S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com