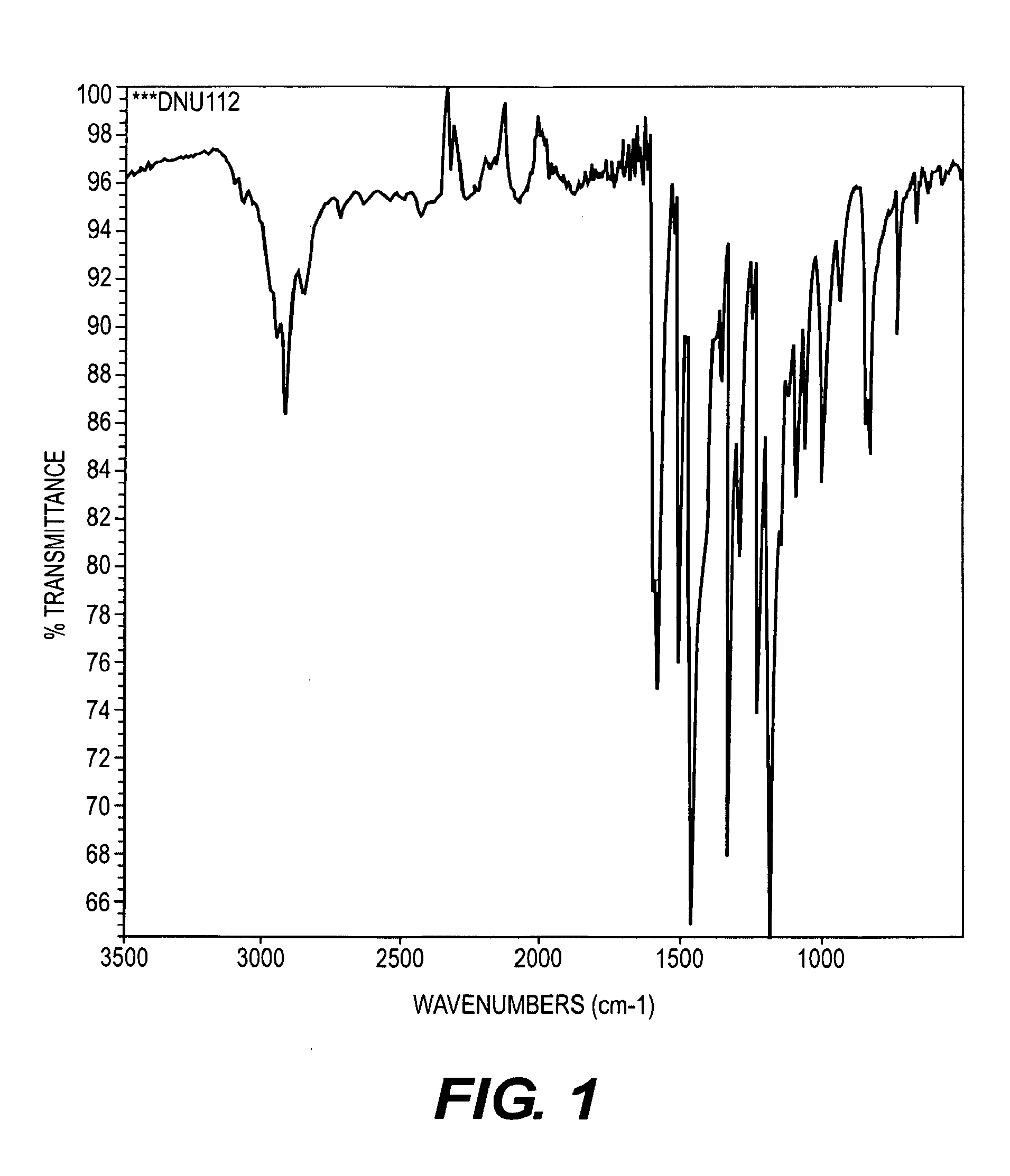
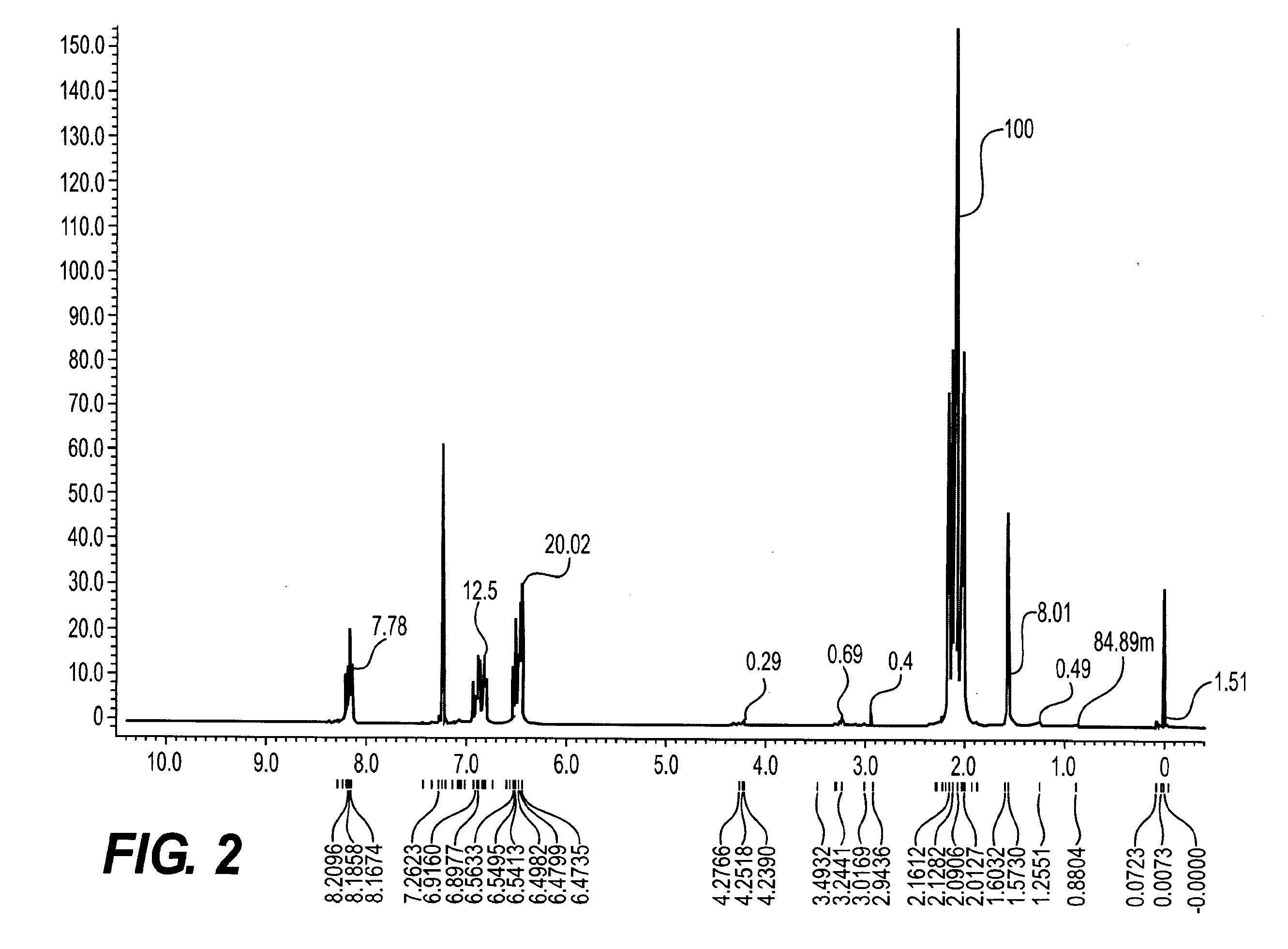
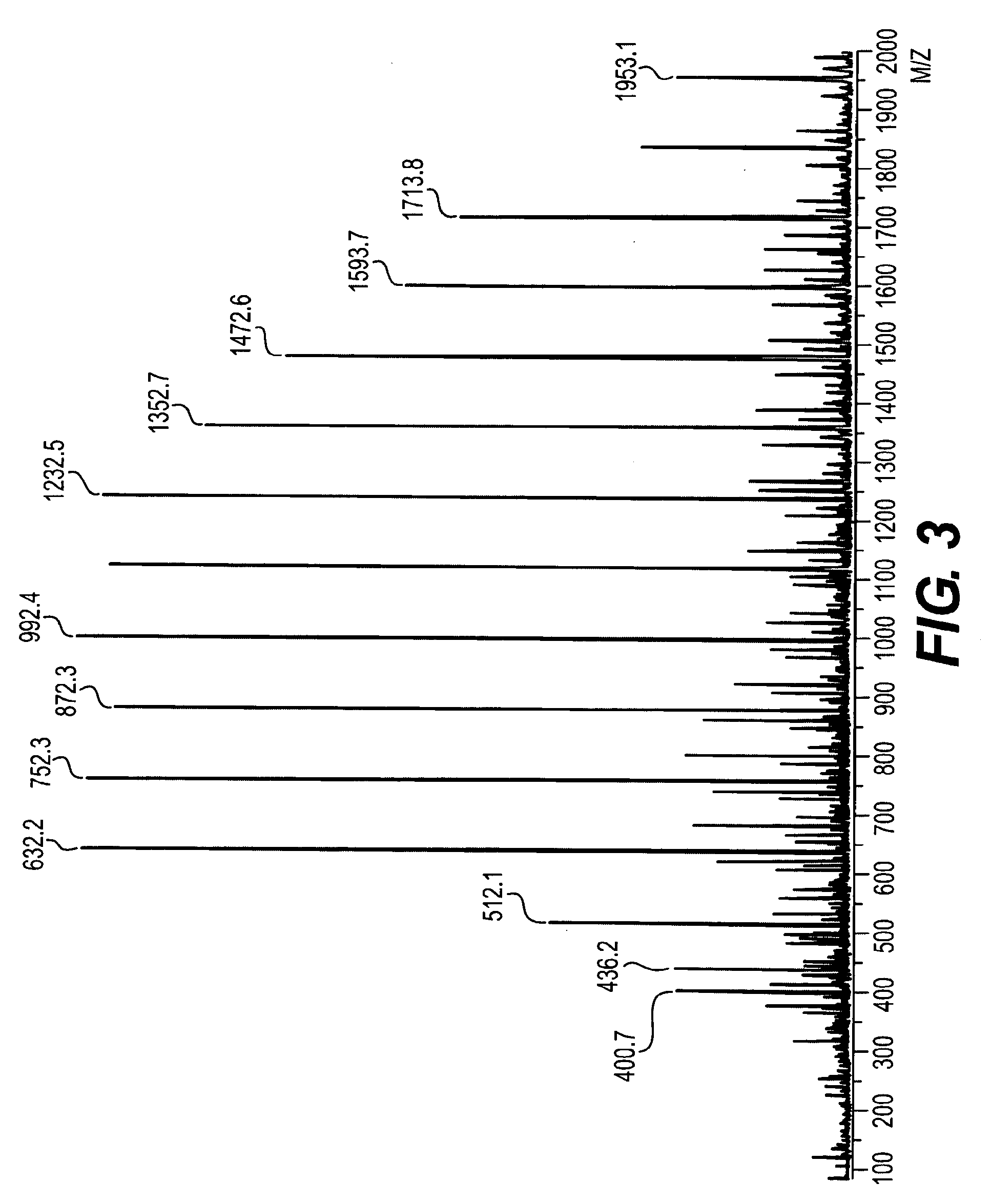
Aromatic diamine compound and aromatic dinitro compound
a technology of aromatic diamine and dinitro compound, which is applied in the preparation of amino compounds, organic chemistry, chemistry apparatus and processes, etc., can solve the problems of aromatic diamine having an oligomer structure, reducing heat resistance, and poor solvent workability, etc., and achieves low water absorption, excellent heat resistance, and low dielectric characteristics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthetic example 1
(Synthesis of Bifunctional Phenylene Ether Oligomer)
[0057]A longitudinally long reactor having a volume of 12 liters and equipped with a stirrer, a thermometer, an air-introducing tube and baffleplates was charged with 3.88 g (17.4 mmol) of CuBr2, 0.75 g (4.4 mmol) of N,N′-di-t-butylethylenediamine, 28.04 g (277.6 mmol) of n-butyldimethylamine and 2,600 g of toluene. The mixture was stirred at a reaction temperature of 40° C. Separately, 129.32 g (0.48 mol) of 2,2′,3,3′,5,5′-hexamethyl-(1,1′-biphenyl)-4,4′-diol, 292.19 g (2.40 mol) of 2,6-dimethylphenol, 0.51 g (2.9 mmol) of N,N′-di-t-butylethylenediamine and 10.90 g (108.0 mmol) of n-butyldimethylamine were dissolved in 2,300 g of methanol, to obtain a mixed solution. The mixed solution was dropwise added to the mixture in the reactor over 230 minutes with stirring. During the above addition of the mixed solution, bubbling was continuously carried out with a nitrogen-air mixed gas having an oxygen concentration of 8% at a flow velo...
synthetic example 2
(Synthesis of Bifunctional Phenylene Ether Oligomer)
[0058]A longitudinally long reactor having a volume of 12 liters and equipped with a stirrer, a thermometer, an air-introducing tube and baffleplates was charged with 9.36 g (42.1 mmol) of CuBr2, 1.81 g (10.5 mmol) of N,N′-di-t-butylethylenediamine, 67.77 g (671.0 mmol) of n-butyldimethylamine and 2,600 g of toluene. The mixture was stirred at a reaction temperature of 40° C. Separately, 129.32 g (0.48 mol) of 2,2′,3,3′,5,5′-hexamethyl-(1,1′-biphenyl)-4,4′-diol, 878.4 g (7.2 mol) of 2,6-dimethylphenol, 1.22 g (7.2 mmol) of N,N′-di-t-butylethylenediamine and 26.35 g (260.9 mmol) of n-butyldimethylamine were dissolved in 2,300 g of methanol, to obtain a mixed solution. The mixed solution was dropwise added to the mixture in the reactor over 230 minutes with stirring. During the above addition of the mixed solution, bubbling was continuously carried out with a nitrogen-air mixed gas having an oxygen concentration of 8% at a flow veloc...
synthetic example 3
(Synthesis of Bifunctional Phenylene Ether Oligomer)
[0059]A longitudinally long reactor having a volume of 12 liters and equipped with a stirrer, a thermometer, an air-introducing tube and baffleplates was charged with 13.1 g (0.12 mol) of CuCl, 707.0 g (5.5 mol) of di-n-butylamine and 4,000 g of methyl ethyl ketone. The mixture was stirred at a reaction temperature of 40° C. A solution of 410.2 g (1.6 mol) of 4,4′-methylenebis(2,6-dimethylphenol) and 586.5 g (4.8 mol) of 2,6-dimethylphenol in 8,000 g of methyl ethyl ketone was dropwise added to the mixture in the reactor over 120 minutes with stirring. During the above addition of the solution, bubbling was continuously carried out with 2 L / min of air. A disodium dihydrogen ethylenediamine tetraacetate aqueous solution was added the stirred mixture to terminate the reaction. Then, washing was three times carried out with 1N hydrochloric acid aqueous solution and then washing was carried out with ion-exchanged water. The thus-obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com