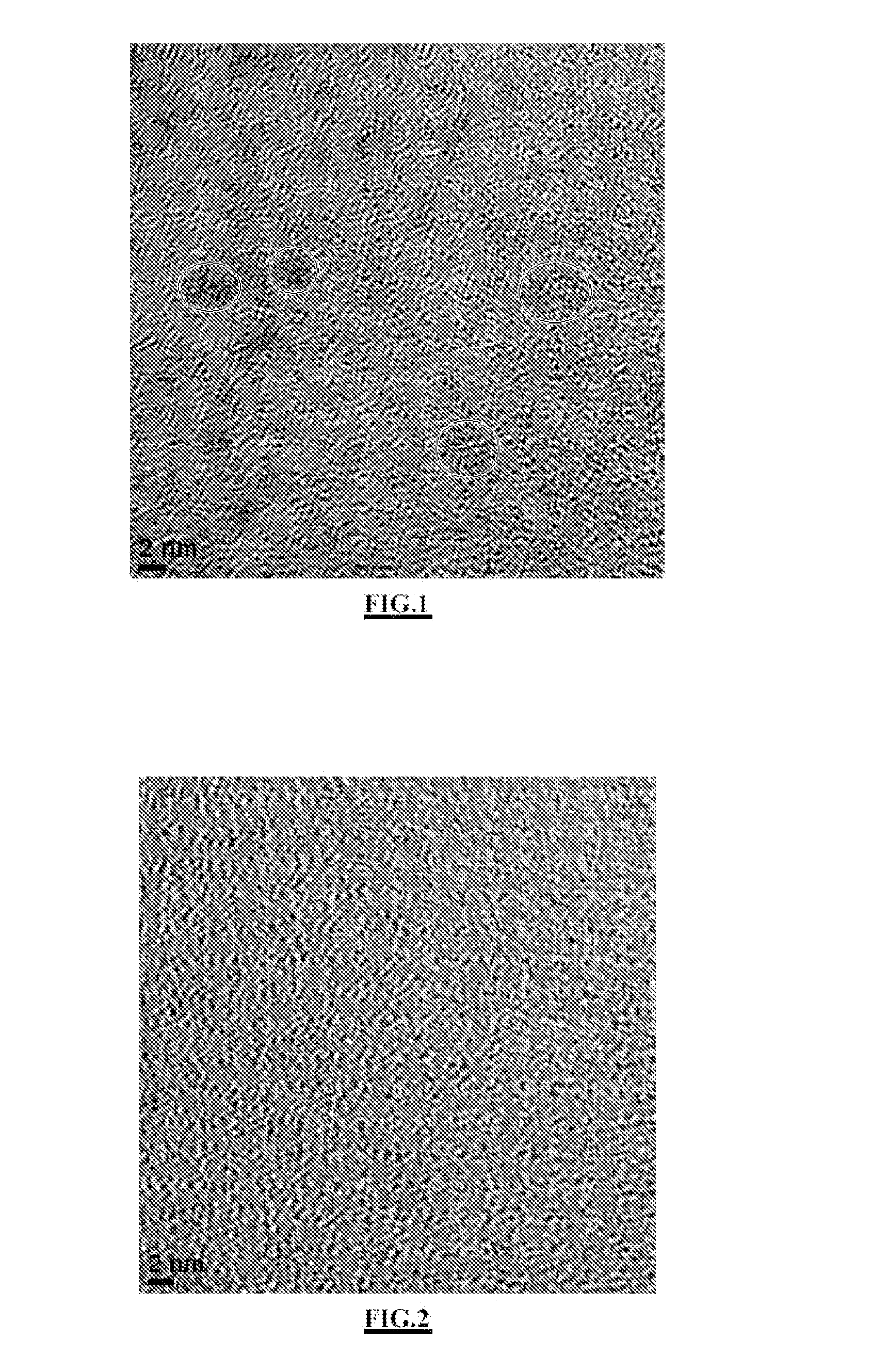
Ultrafine nanoparticles comprising a functionalized polyorganosiloxane matrix and including metal complexes; method for obtaining same and uses thereof in medical imaging and/or therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of the Gadolinium Oxide Core
[0260]A solution is prepared by dissolving a quantity of 11 g / L of gadolinium chloride salt (GdCl3, 6H2O) in a volume of 375 mL of diethylene glycol (DEG). 375 mL of a soda solution at 0.1 mol / L in DEG is added to the solution obtained, at room temperature, in 15 h.
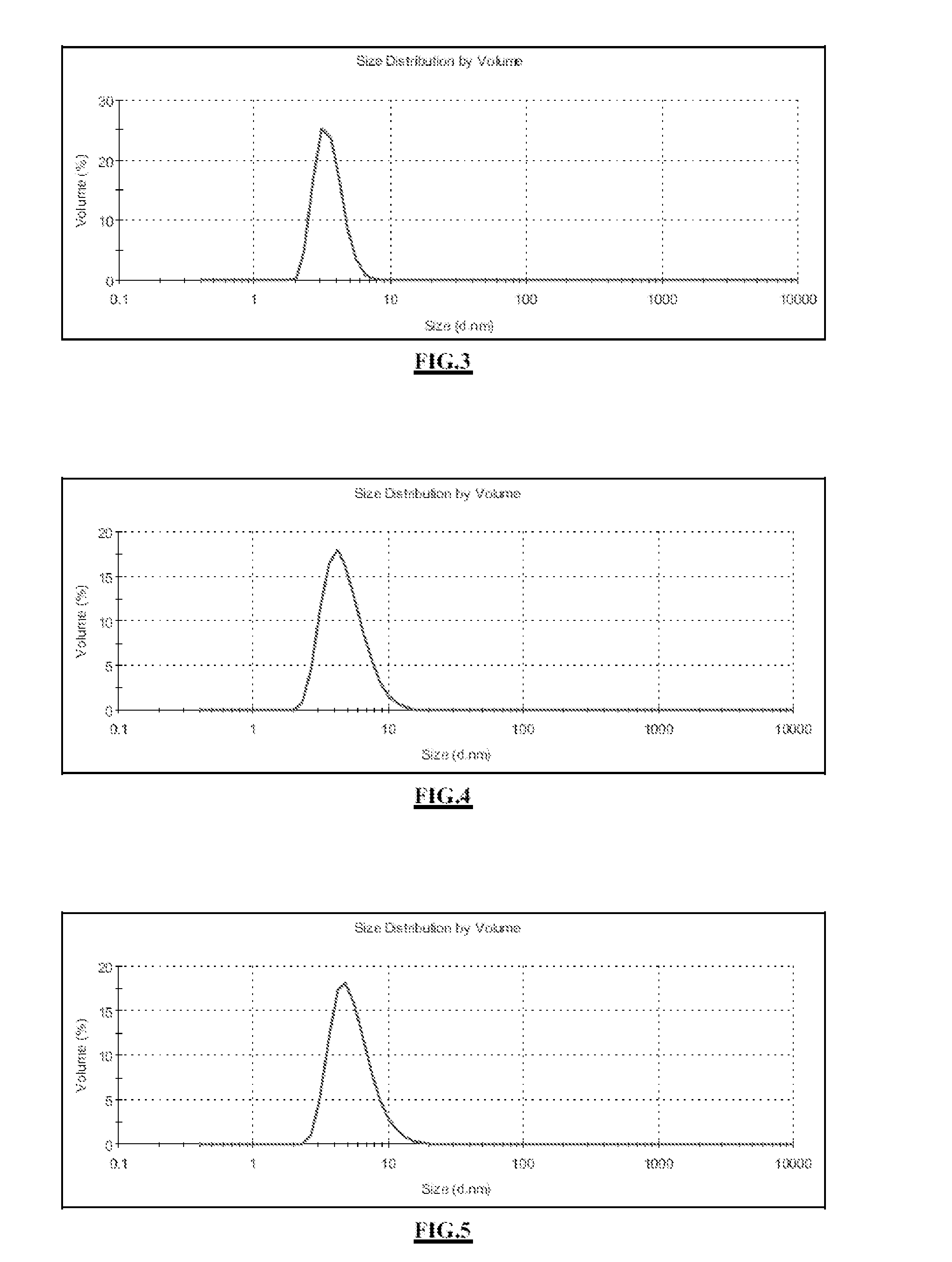
[0261]The attached FIG. 3 shows the size distribution of the gadolinium oxide cores, measured in DEG by PCS; mean value: 3.6 nm.
example 2
Functionalization of the Gadolinium Oxide Cores with DTPABA
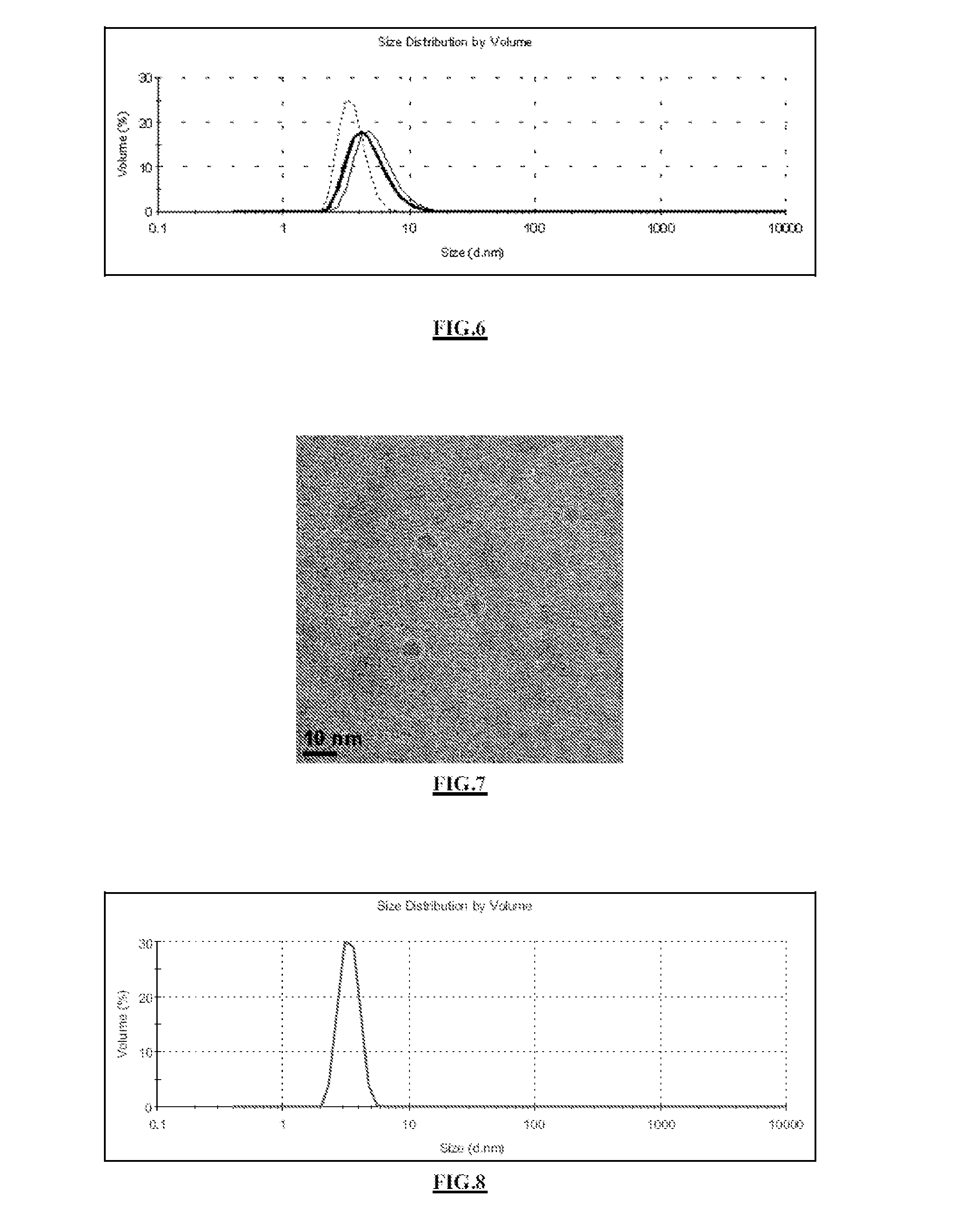
[0262]A layer of functionalized polysiloxane is synthesized by the sol-gel process around the gadolinium oxide cores from Example 1. For this purpose, the 750 mL solution of cores is heated to 40° C. in an oil bath, with stirring. 787 μL of APTES, 502 μL of TEOS and 1913 μL of an aqueous solution of triethylamine at 0.1 mol / L are added to the solution of cores. These additions are repeated a second time after waiting 24 h. The solution is then stirred at 40° C. for 48 h. Core-shell particles are obtained with a size of about 5 nm, with amine functions on the surface, FIG. 4 shows the size distribution of the polysiloxane-coated cores, measured in DEG by PCS; mean value: 4.9 nm.
[0263]Then 3.135 g of DTPABA is dispersed in 150 mL of dimethyl sulphoxide (DMSO). Then the 750 mL of solution of gadolinium oxide cores is added to the solution of DTPABA. The mixture is stirred for 24 h.
[0264]The nanoparticles are then precipitated b...
example 3
Dissolution of the Core of DTPABA-Functionalized Gadolinium Oxide Particles by Means of Hydrochloric Acid
[0270]Concentrated hydrochloric acid is added to the particles from Example 2, dispersed in water, until a pH equal to 2.5 is obtained. It is stirred overnight.
[0271]The solution is purified by tangential filtration, to remove the Gd3+ ions that were dissolved from the core of the particles. Particles are obtained with a size of about 3.5 nm. The attached FIG. 8 shows the size distribution of the particles reduced in size with hydrochloric acid, measured in water by PCS; mean value: 3.4 nm.
[0272]The signal from the particles in relaxornetry r1=1 / T1 decreased by 27%. Considerable chemical attack was observed with forced dissolution by acid attack. This is confirmed by the large decrease in relaxometry.
[0273]Moreover, chemical analysis by EDX gives a gadolinium / silicon atomic ratio of 22.1%. Relative to the particles from Example 2, it is observed that 54% of the gadolinium was dis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Force | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com