Polar group-containing olefin copolymer, multinary polar olefin copolymer, olefin resin composition, and adhesive material, laminate, and other application products using same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1-1
Production of Drent Catalyst and Polymerization Therewith
[0257]In a fully nitrogen-purged 30-mL flask, palladium bisdibenzylideneacetone and phosphorus-sulfonic acid ligand (I) were weighed each in an amount of 100 μmol, then dewatered toluene (10 mL) was added thereto and processed with an ultrasonic vibrator for 10 minutes to prepare a catalyst slurry.
[0258]Next, a stainless autoclave having an internal volume of 2.4 liters and equipped with an induction stirrer was purged with pure nitrogen, and pure toluene and 5-norbornene-2,3-dicarboxylic acid anhydride were introduced into the autoclave in a pure nitrogen atmosphere in such a manner that the polar group-containing monomer concentration therein could be 0.1 mol / L.
[0259]The previously prepared catalyst solution was added thereto, and the polymerization was started at 100° C. and under an ethylene pressure of 1 MPa. During the reaction, the temperature was kept at 100° C., and ethylene was continuously fed so as to maintain the ...
examples 1-2 to 1-11 , 1-14
Examples 1-2 to 1-11, 1-14, Comparative Examples 1-1 to 1-6
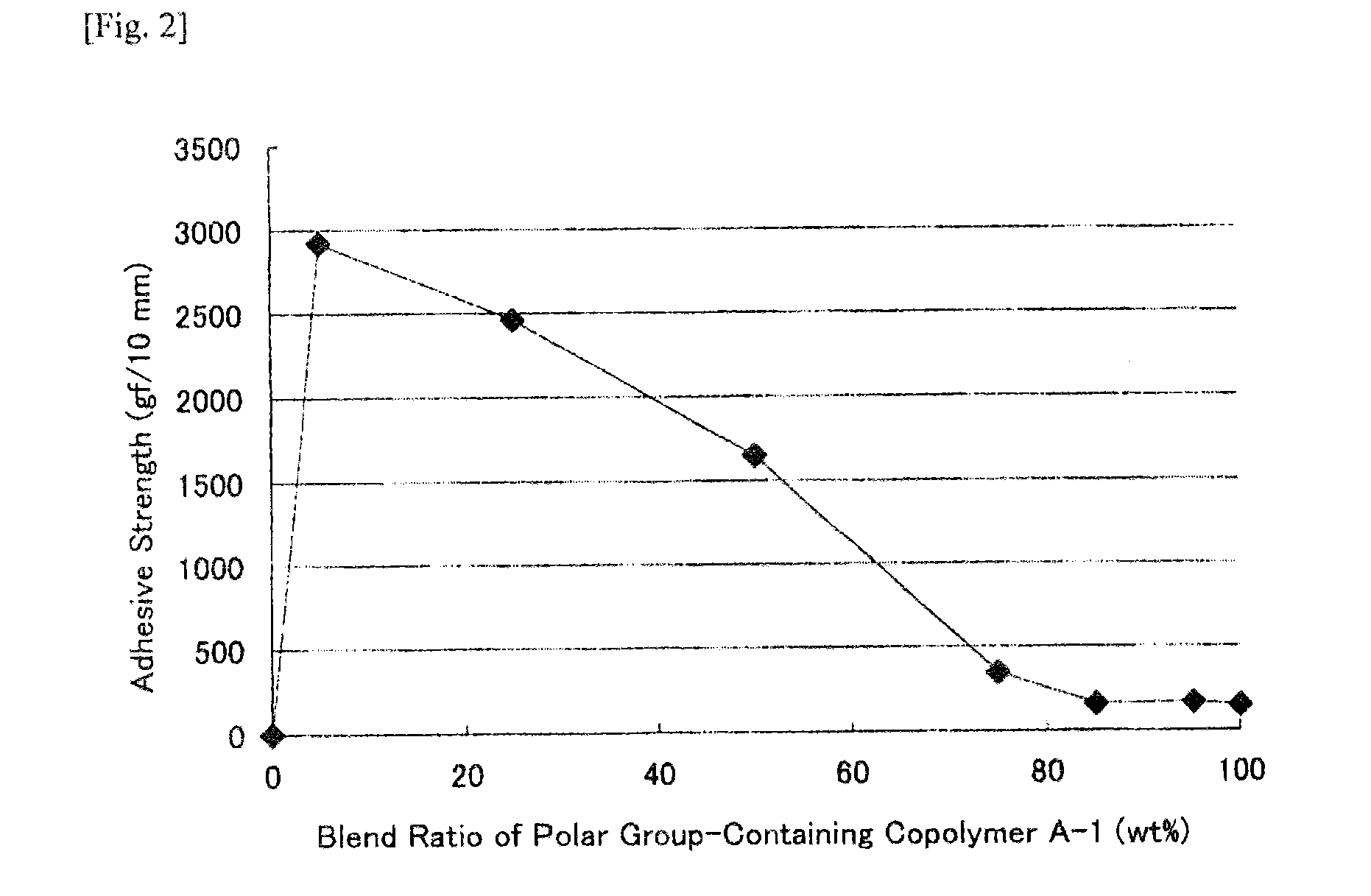
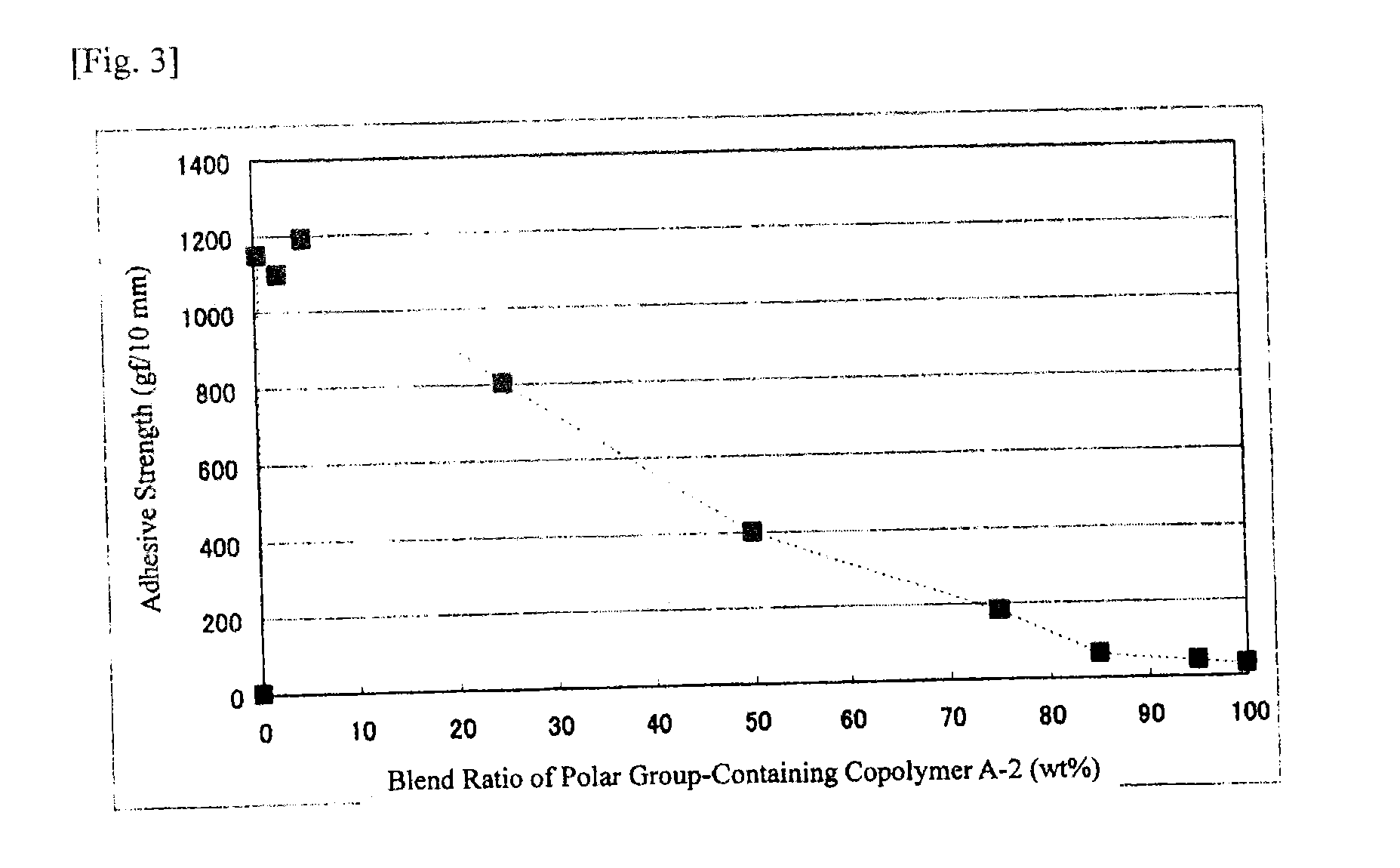
[0261]Polar group-containing olefin copolymers of Examples 1-2 to 1-11, Example 1-14 and Comparative Examples 1-1 to 1-6 were prepared through polymerization according to the method of Example 1-1 except that the ligand species, the ligand amount, the polar group-containing monomer species, the polar group-containing monomer concentration, the polymerization pressure, the polymerization temperature and the polymerization time were changed. The polymerization condition and the activity are shown in Table 1. The chemical formulae of the ligand species (I) to (IV) in Table 1 are shown below. The analysis data of the polar group-containing olefin copolymers obtained in Examples and Comparative Examples, and the adhesive strength of the EVOH film are shown in Table 2. In Table 2, the end introduction means the polar group containing structural unit amount of the polar group-containing monomer introduced into the end of the copoly...
example 1-12
Synthesis of SHOP Ligand
[0262]The following ligand B-27DM was obtained according to the method described in WO2010 / 050256 (Synthesis Example 4).
Formation of Complex:
[0263]First, in a 50-ml eggplant flask, 112 mg (200 μmol) of the above B-27DM was weighed and put. Next, bis-1,5-cyclooctadiene nickel(0) (hereinafter referred to as Ni(COD)2) was weighed and put in a 50-ml eggplant flask, and dissolved in 20 ml of dry toluene to prepare a 10 mmol / l Ni(COD)2 / toluene solution. The whole amount (20 ml) of the Ni(COD)2 / toluene solution prepared here was added to the eggplant flask containing B-27DM therein, and stirred in a hot-water bath at 40° C. for 30 minutes to give 20 ml of a 10 mmol / l solution of a reaction product of B-27DM and Ni(COD)2.
[0264]Next, a stainless autoclave having an internal volume of 2.4 liters and equipped with an induction stirrer was purged with pure nitrogen, and pure toluene (1.0 L) and 5-norbornene-2,3-dicarboxylic acid anhydride (8.2 g) were introduced into the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com