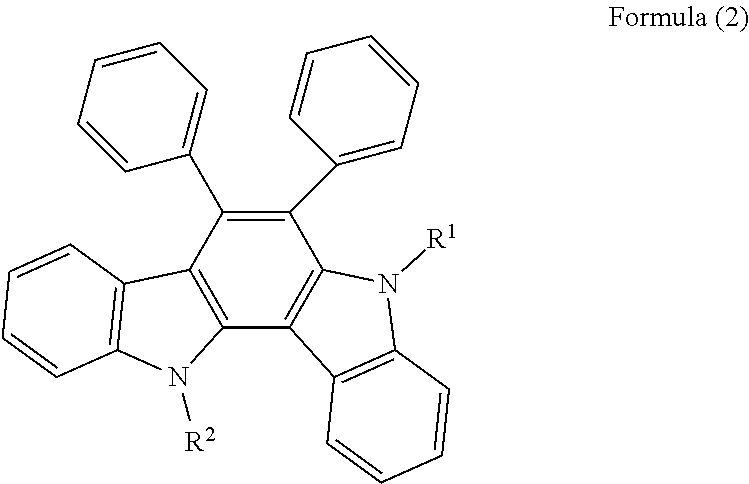
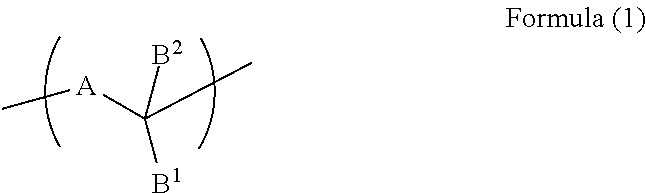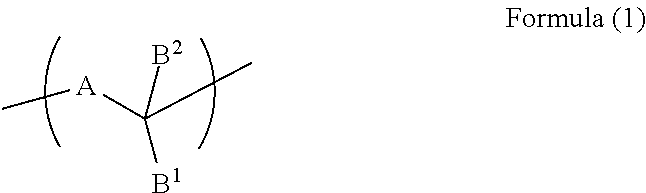Resist underlayer film-forming composition containing indolocarbazole novolak resin
a technology of indolocarbazole and film-forming composition, which is applied in the direction of photomechanical equipment, photosensitive material processing, instruments, etc., can solve the problems of severe standing wave effects, resist pattern collapse, and resist pattern collapse, so as to achieve sufficient etching resistance, efficient suppression of reflection from the substrate, and favorable pattern shape of resis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
[0090]In a three-neck flask, 5.00 g of the compound 1, 2.84 g of 1-pyrenecarboxyaldehyde (available from Aldrich), 19.12 g of propylene glycol monomethyl ether acetate, and 0.35 g of methanesulfonic acid (available from Tokyo Chemical Industry Co., Ltd.) were placed. The mixture was then heated to 150° C., and stirred under reflux for about 36 hours. After completion of the reaction, the mixture was diluted with 24.80 g of propylene glycol monomethyl ether acetate, and the precipitate was removed by filtration. The collected filtrate was added dropwise to a methanol solution, resulting in reprecipitation. The obtained precipitate was filtered by suction, and the filtered product was dried at 60° C. overnight under reduced pressure. As a result, 2.51 g of resin was obtained as a black powder. The resulting polymer corresponded to formula (1-1). The weight average molecular weight Mw measured by GPC in terms of polystyrene was 5,900 and the polydispersity index Mw / Mn was 2.19.
synthesis example 2
[0091]In a two-neck flask, 8.00 g of the compound 1, 2.08 g of benzaldehyde (available from KISHIDA CHEMICAL Co., Ltd.), 28.39 g of propylene glycol monomethyl ether acetate, 7.10 g of N-methyl-2-pyrrolidinone (available from KANTO CHEMICAL CO., INC.), and 1.13 g of methanesulfonic acid (available from Tokyo Chemical Industry Co., Ltd.) were placed. The mixture was then heated to 150° C., and stirred under reflux for about 20 hours. After completion of the reaction, the mixture was diluted with 20.49 g of propylene glycol monomethyl ether acetate, and the precipitate was removed by filtration. The collected filtrate was added dropwise to a methanol solution, resulting in reprecipitation. The obtained precipitate was filtered by suction, and the filtered product was dried at 60° C. overnight under reduced pressure. As a result, 6.42 g of resin was obtained as a gray powder. The resulting polymer corresponded to formula (1-2). The weight average molecular weight Mw measured by GPC in ...
synthesis example 3
[0092]In a two-neck flask, 8.00 g of the compound 1, 3.06 g of 1-naphthaldehyde (available from Tokyo Chemical Industry Co., Ltd.), 32.83 g of propylene glycol monomethyl ether acetate, 8.21 g of N-methyl-2-pyrrolidinone (available from KANTO CHEMICAL CO., INC.), and 2.26 g of methanesulfonic acid (available from Tokyo Chemical Industry Co., Ltd.) were placed. The mixture was then heated to 150° C., and stirred under reflux for about 20 hours. After completion of the reaction, the mixture was diluted with 19.37 g of propylene glycol monomethyl ether acetate, and the precipitate was removed by filtration. The collected filtrate was added dropwise to a methanol solution, resulting in reprecipitation. The obtained precipitate was filtered by suction, and the filtered product was dried at 60° C. overnight under reduced pressure. As a result, 6.58 g of resin was obtained as a gray powder. The resulting polymer corresponded to formula (1-3). The weight average molecular weight Mw measured...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com