Inhibitor of RNA polymerase ii
a technology of rna polymerase and inhibitor, which is applied in the field of inhibitors of rna polymerase ii to achieve the effect of reducing the expression of proteins and increasing the phosphorylation of host pol ii
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Identification of an ABU 83972 Variant SN25 with Loss of Pol II Inhibitory Activity and its Genome Sequencing
[0057]Patients were inoculated with therapeutic doses of ABU 83972. The protocol for therapeutic bladder inoculation of patients with E. coli 83972 has been described previously (Agace, J Clin Invest, 1993; Wullt, Mol Microbiol, 2000; Sunden, J Urol, 2010). Briefly, after antibiotic treatment to remove prior infection, patients were inoculated with E. coli 83972 through a catheter (30 ml, 105 cfu / ml in saline). Blood and urine samples were obtained before and repeatedly after inoculation. Throughout the colonization period, viable bacterial counts in urine were determined, monthly urine samples were collected and analyzed for IL-6 and IL-8 as well as neutrophil infiltration. Bacteria from each urine sample were verified by PCR for presence of a kryptic plasmid unique for strain 83972 and one chromosomal marker (4.7-kb deletion in strain 83972 in the type 1 fimbrial gene clust...
example 3
Screening of Single-Gene Mutants to Identify Genes Responsible for Pol II Inhibition
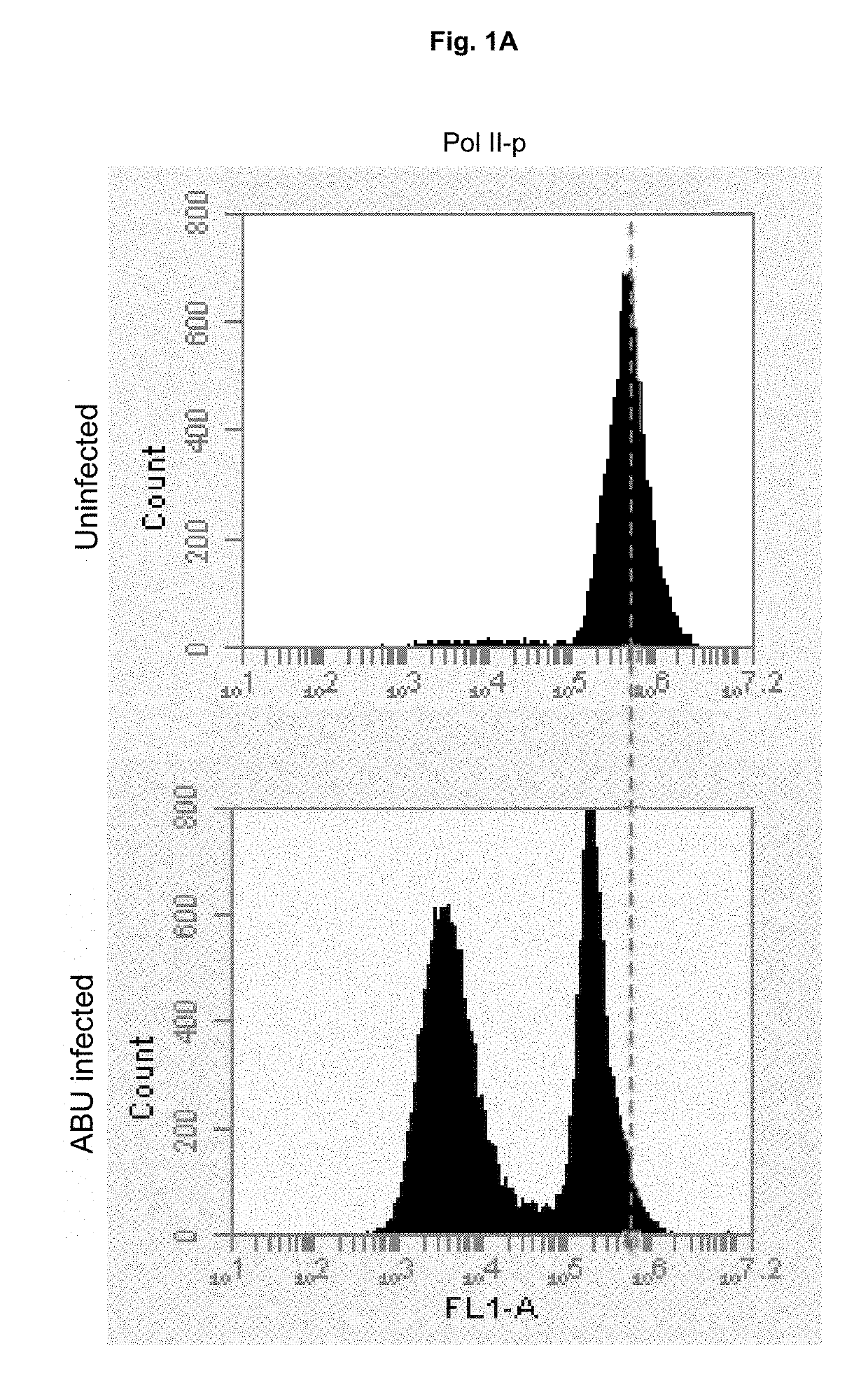
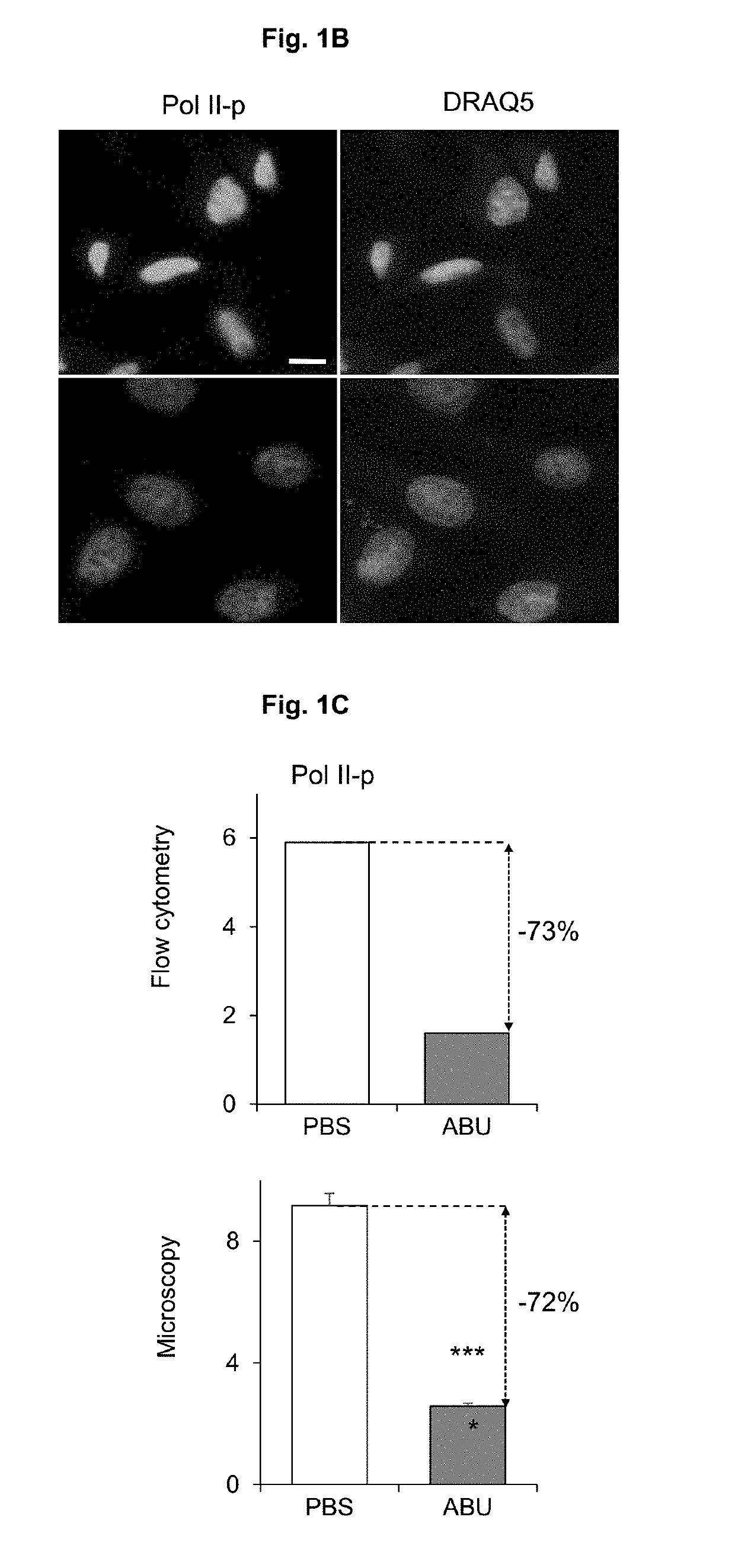
[0060]To identify genetic determinants of Pol II phosphorylation, genes comprising the identified variant sequences were replaced in E. coli 83972 chromosome by homologous recombination with chloramphenicol resistance cassette. Deletions were validated (Uli). The mutants were subsequently screened for effects on Pol II (FIG. 3A). Single deletions of ΔlldD, ΔlldR, ΔnlpD, ΔrfaH and ΔcysE reduced the inhibitory effect of E. coli 83972 wt, as shown by flow cytometry and confocal microscopy (FIG. 3B-C). Statistical difference in level of Pol II phosphorylation was measured with t test.
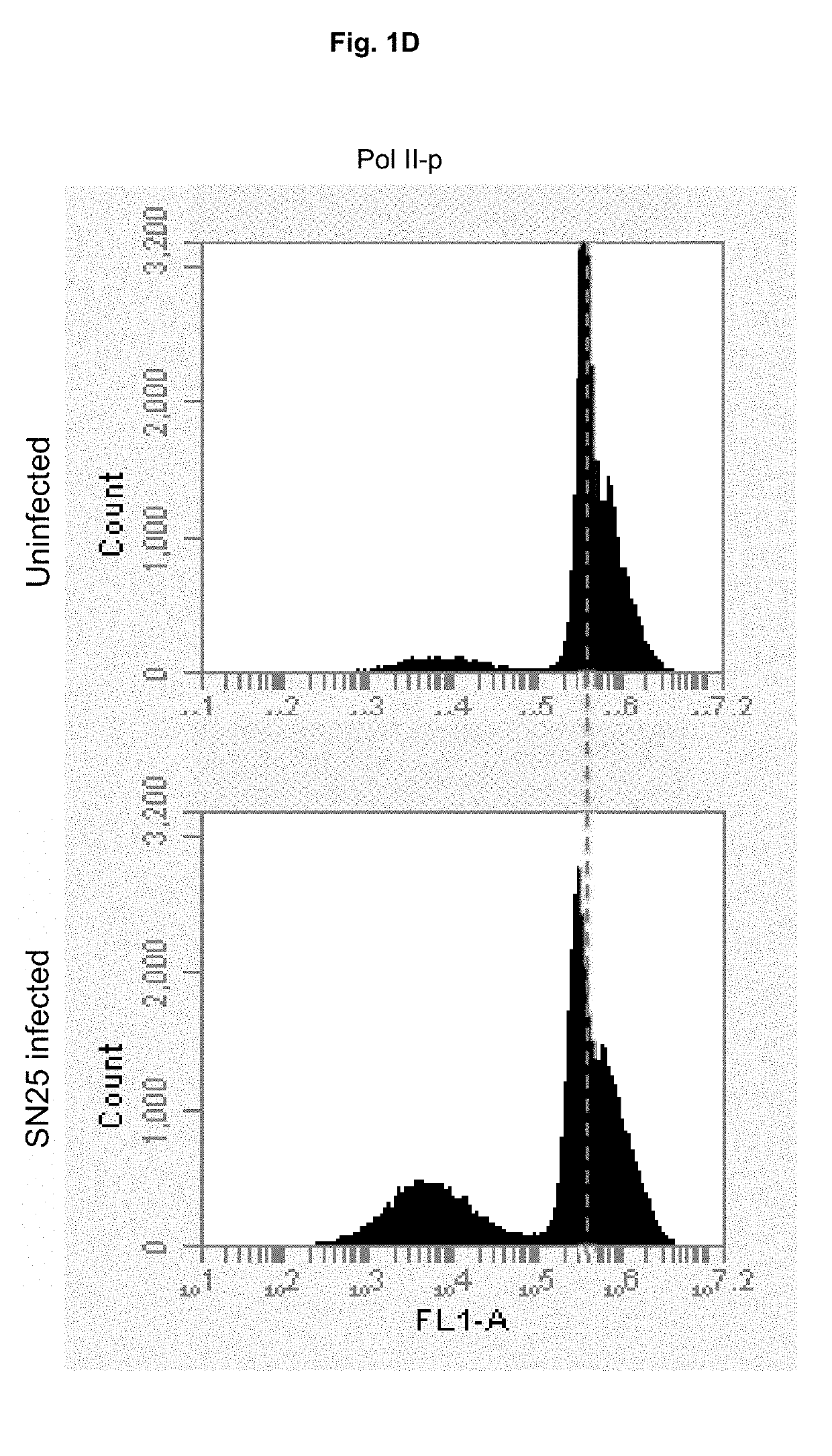
[0061]The lldD gene is responsible for aerobic L-lactate metabolism, whose product catalyzes the interconversion of L-lactate and pyruvate, while lldR is a regulator of the lldPRD operon. It was concluded from these data that products of both lldD and lldR genes are responsible for suppression of RNA Polymerase II phosphoryl...
example 4
Secretion of Bacterial Inhibitors of Pol II Phosphorylation
[0063]In parallel with the genetic studies, a biochemical approach was taken to identify the compound responsible for the Pol II inhibitory activity. Bacteria were incubated for 4 hours in tissue culture medium (RPMI supplemented with 1 mM pyruvate). The medium was harvested after 4 hours, centrifuged at 4,000×g for 10 min and sterile filtered to remove remaining bacteria (0.2 μm filter), before addition to human kidney cells (FIG. 3D). E. coli are typically 2 μm long and 0.5 μm in diameter, and filtration through 0.2 μm syringe filters removes bacterial cells. Significant Pol II inhibitory activity (p=0.023) was identified in the ABU culture supernatants compared to uninfected, substituted RPMI, suggesting that growth in cell-free culture medium induced the secretion of the inhibitor(s).
[0064]As it was shown that supernatant of ABU bacteria have similar inhibitory effect on Pol II as ABU bacteria per se, we questioned if th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com