Carbon aerogels for supercapacitors and method of manufacturing the same
a supercapacitor and aerogel technology, applied in chemical/physical processes, non-conductive materials with dispersed conductive materials, synthetic resin layered products, etc., can solve the problem that existing methods need an additional activation step to obtain high activity, and achieve the effect of improving time efficiency and energy efficiency, simplifying the overall process, and shortening the time period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
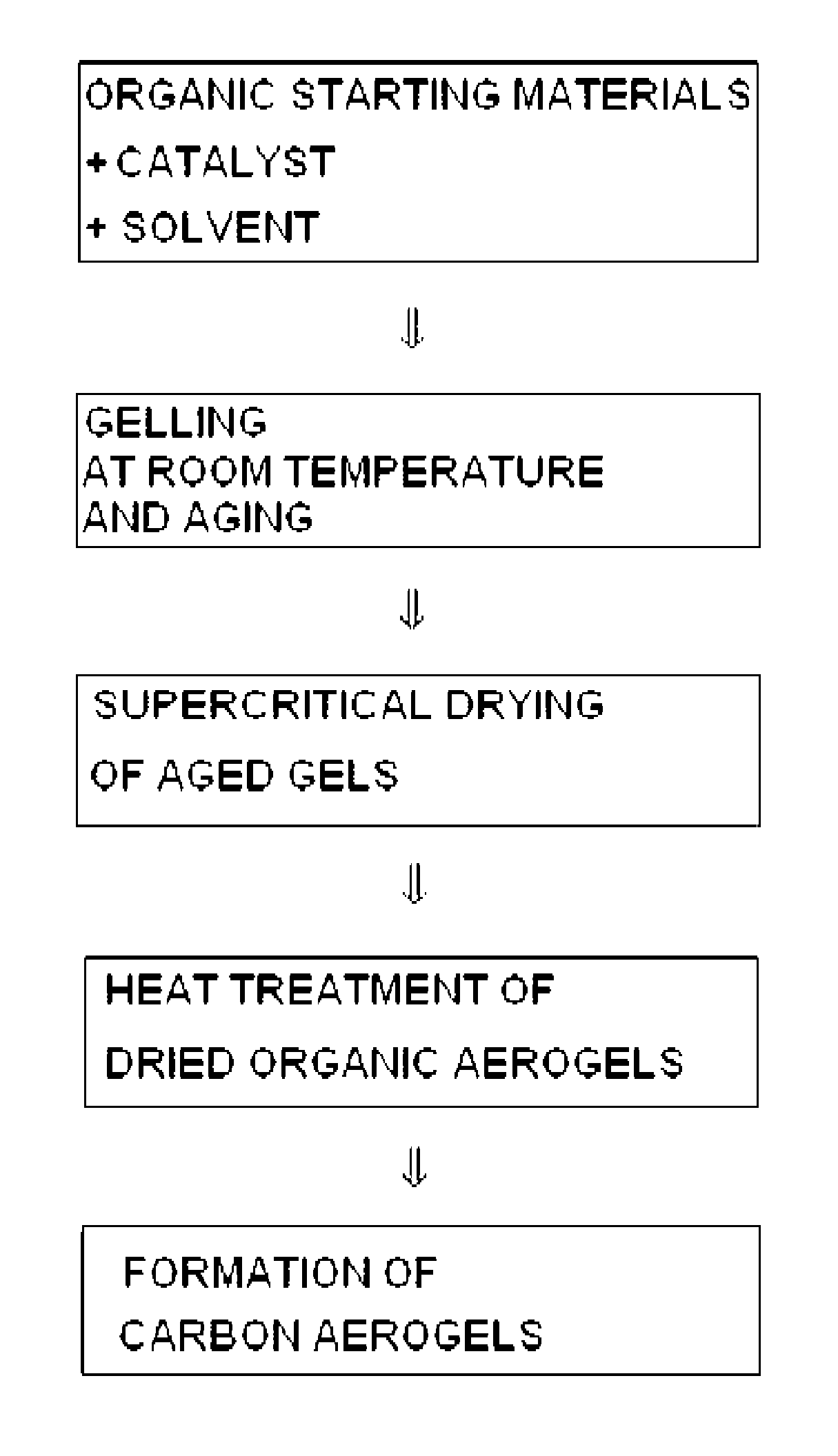
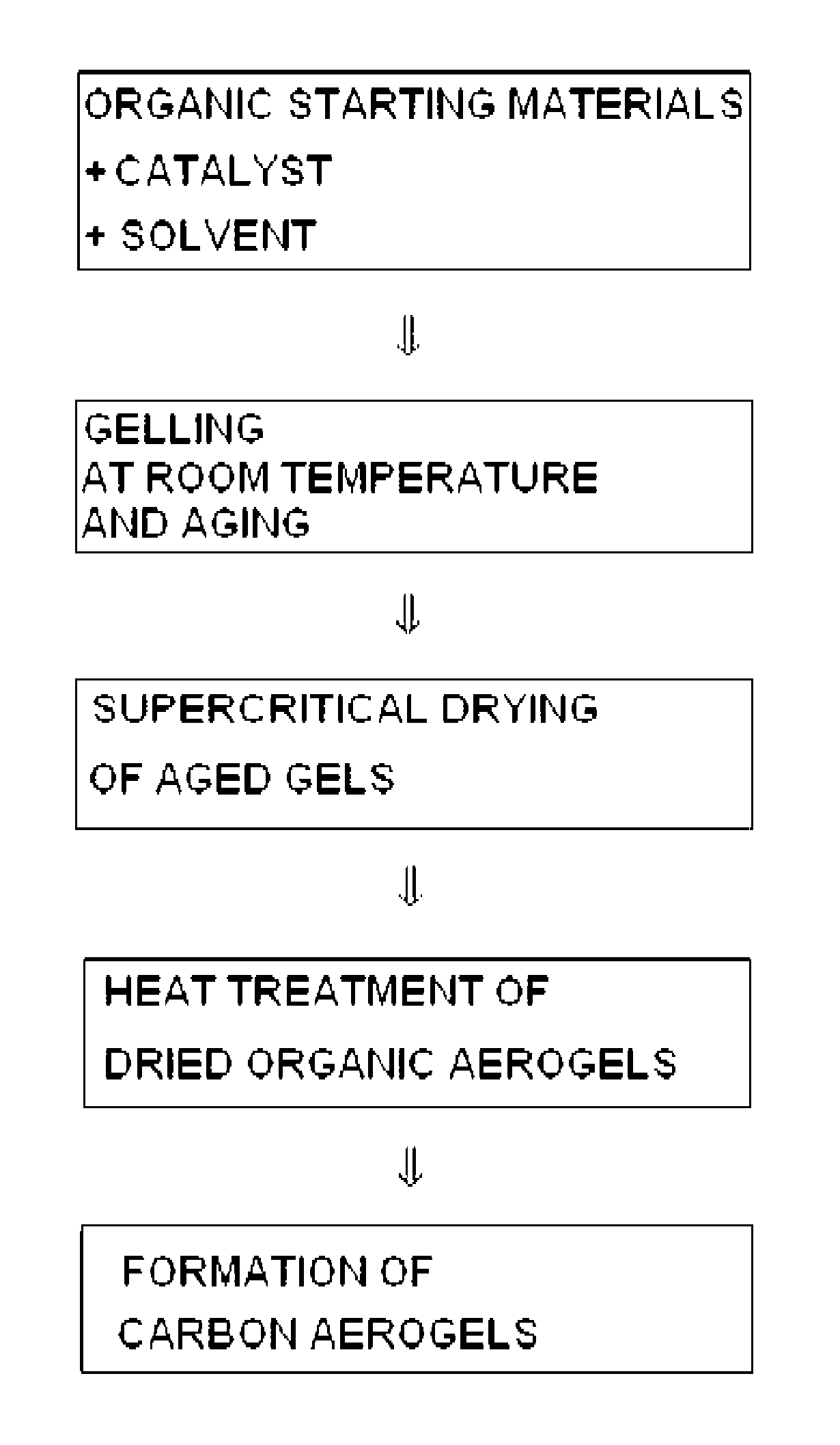
[0019]First, 1.6378 g of phloroglucinol is dissolved in 58.6 mL of ethanol. Next, nitric acid is added thereto in a molar ratio of 1 / 0.001 based on phloroglucinol, and the reaction mixture is sealed and agitated for 30 minutes at room temperature. Then, furfural is added in a molar ratio of 1 / 3 based on phloroglucinol, and the reaction mixture is sealed and agitated at room temperature to carry out a reaction. After gels are formed, agitation is terminated and the gels are aged at room temperature for 4 days. The aged gels are subjected to supercritical drying under 170 atm at 70° C. to form organic aerogels. Finally, the organic aerogels are heat treated in an electric furnace, through which helium flows, at a heating rate of 1° C. / min to 800° C. so that the organic aerogels are converted into carbon aerogels.
example 2
[0021]First, 1.6378 g of phloroglucinol is dissolved in 58.6 mL of ethanol. Next, furfural is added thereto in a molar ratio of 1 / 3 based on phloroglucinol. Then, the reaction mixture is sealed and agitated at room temperature to carry out a reaction. After gels are formed, agitation is terminated and the gels are aged at room temperature for 3 days. The aged gels are subjected to supercritical drying under 170 atm at 70° C. to form organic aerogels. Finally, the organic aerogels are heat treated in an electric furnace, through which helium flows, at a heating rate of 1° C. / min to 800° C. so that the organic aerogels are converted into carbon aerogels.
example 3
[0023]First, 1.6378 g of phloroglucinol is dissolved in 58.6 mL of ethanol. Next, potassium hydroxide is added thereto in a molar ratio of 1 / 0.01 based on phloroglucinol. The reaction mixture is sealed and agitated at room temperature for 30 minutes. Then, furfural is added thereto in a molar ratio of 1 / 3 based on phloroglucinol, and the reaction mixture is sealed and agitated at room temperature to carry out a reaction. After gels are formed, agitation is terminated and the gels are aged at room temperature for 3 days. The aged gels are subjected to supercritical drying under 170 atm at 70° C. to form organic aerogels. Finally, the organic aerogels are heat treated in an electric furnace, through which helium flows, at a heating rate of 1° C. / min to 800° C. so that the organic aerogels are converted into carbon aerogels.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com