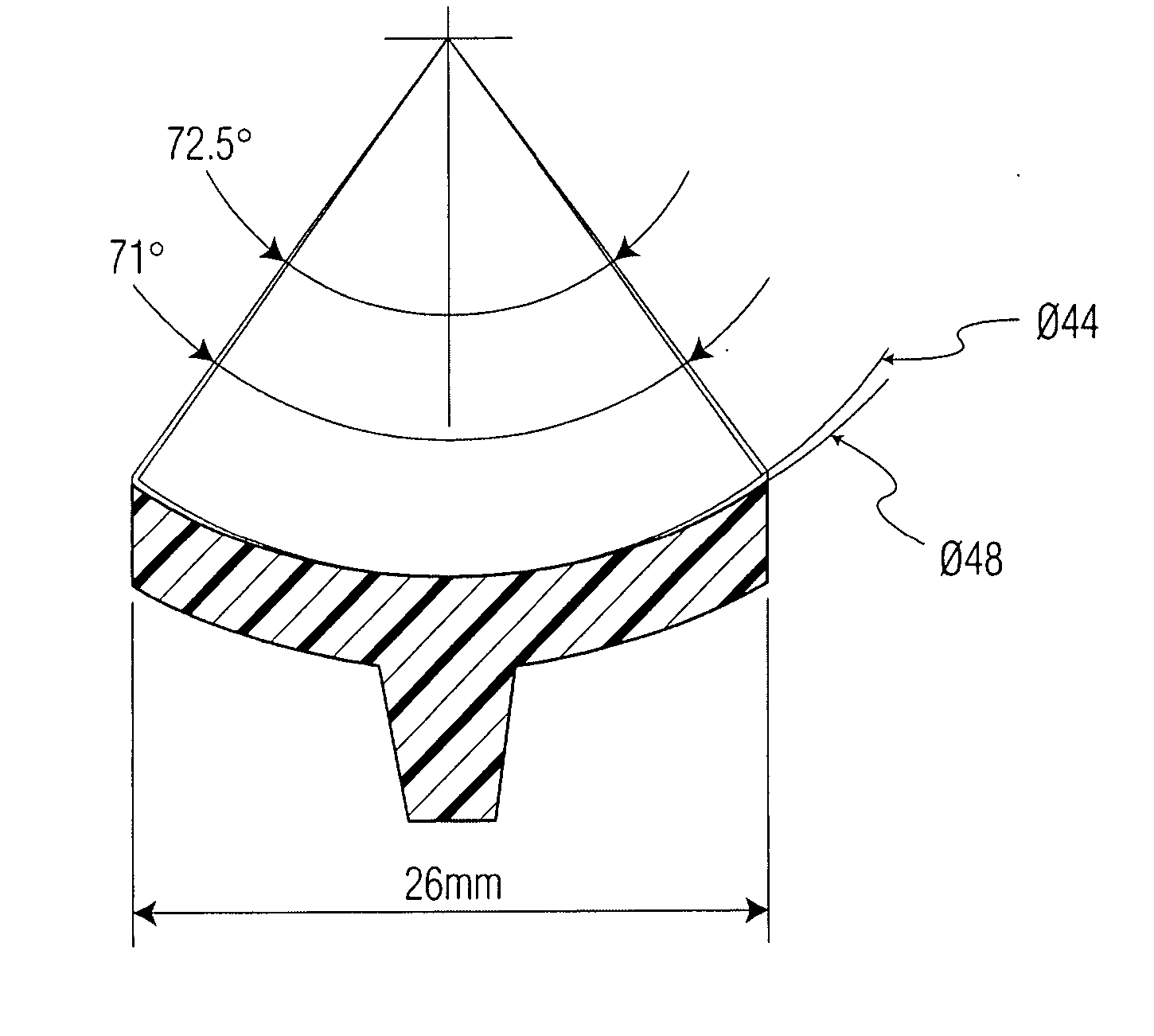
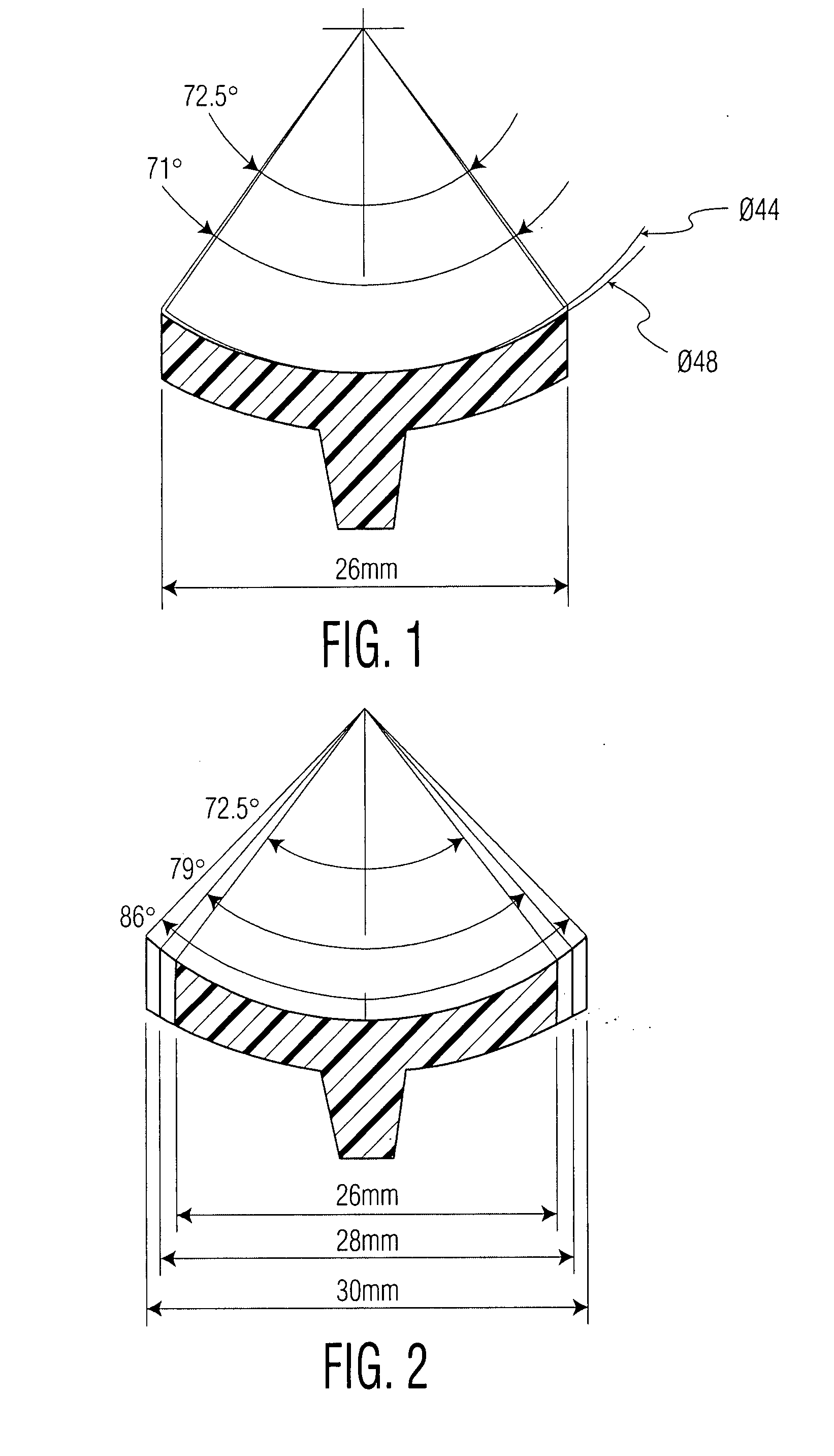
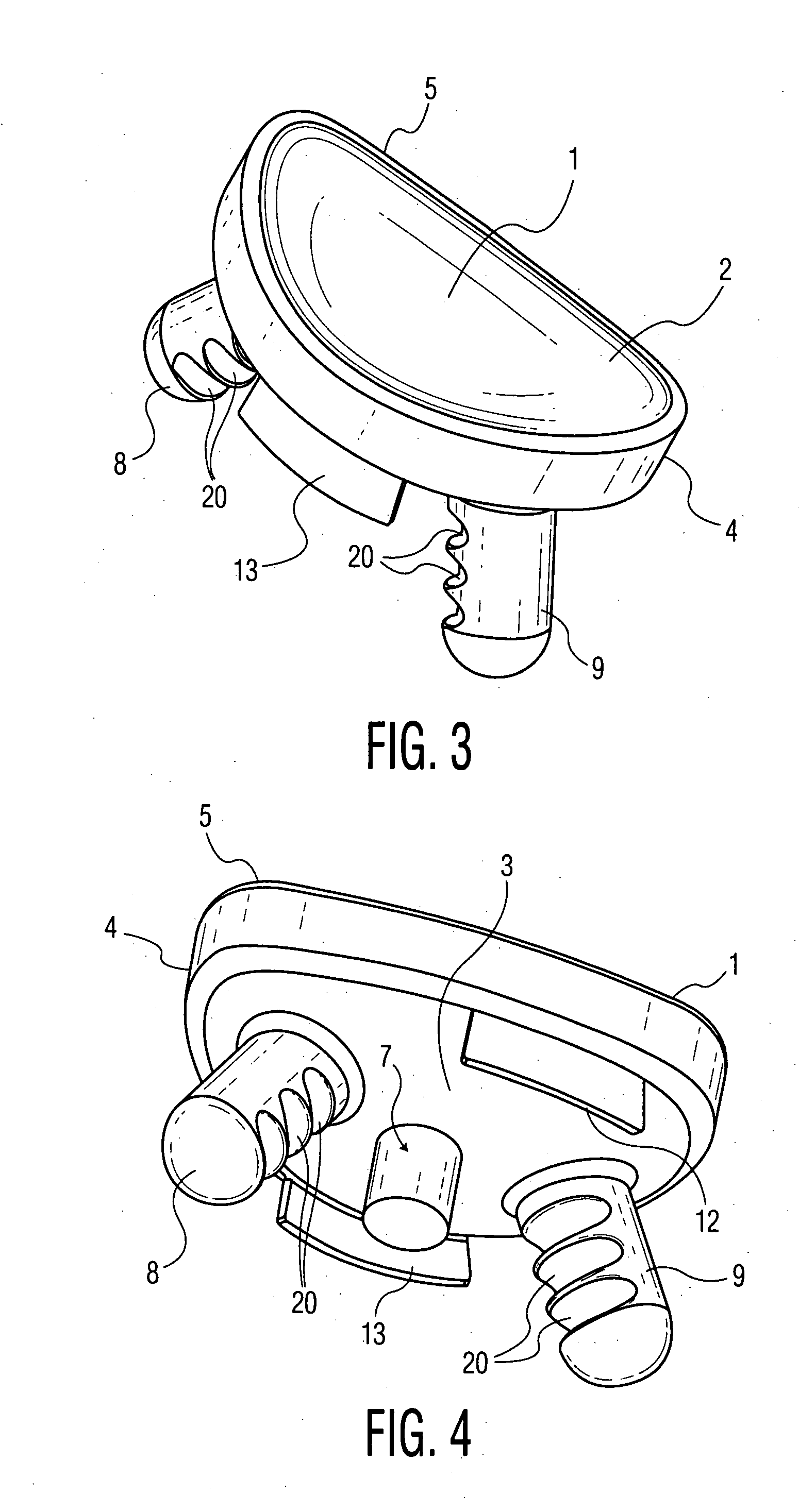
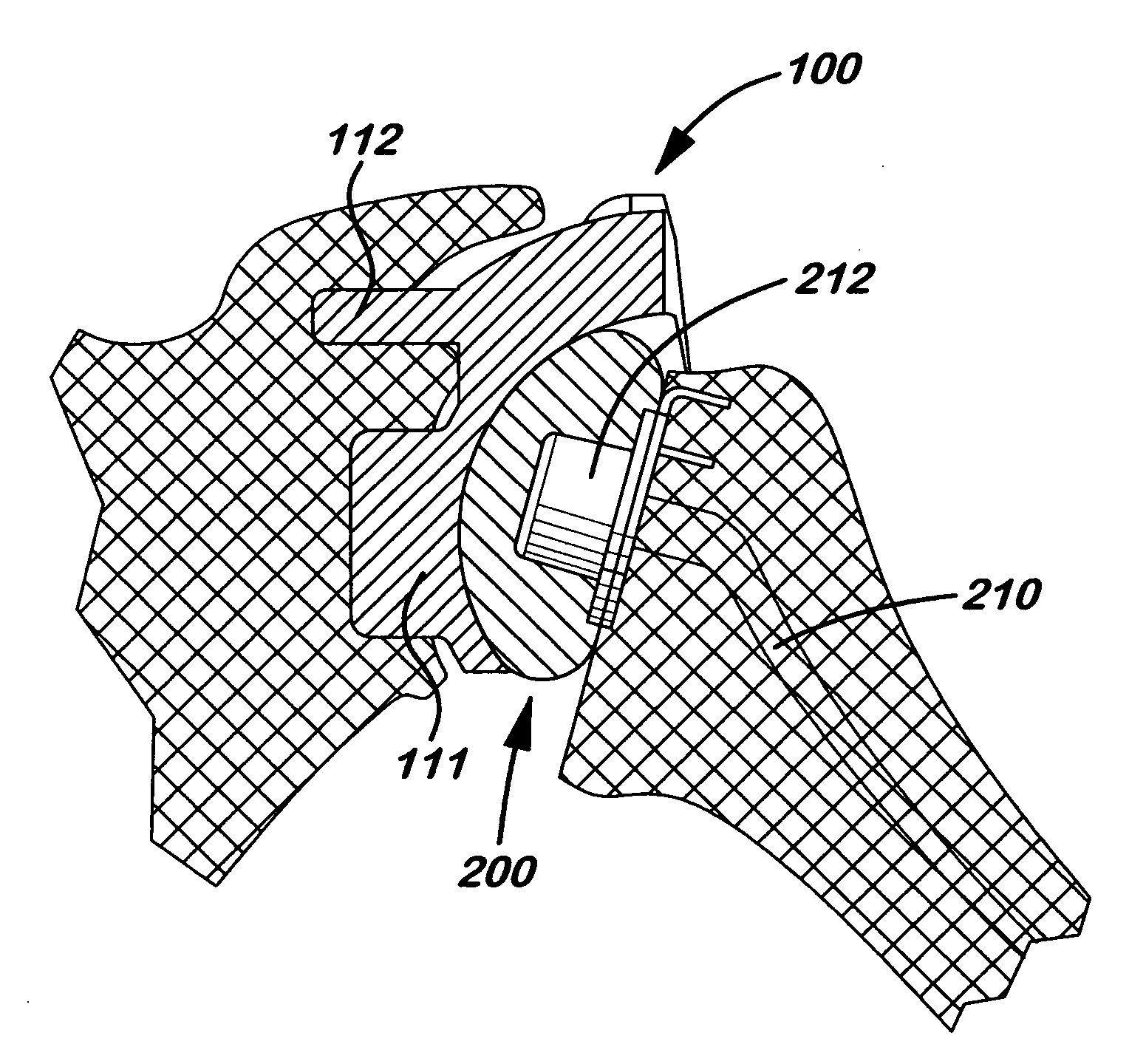
Patents
Literature
55 results about "Glenoid component" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
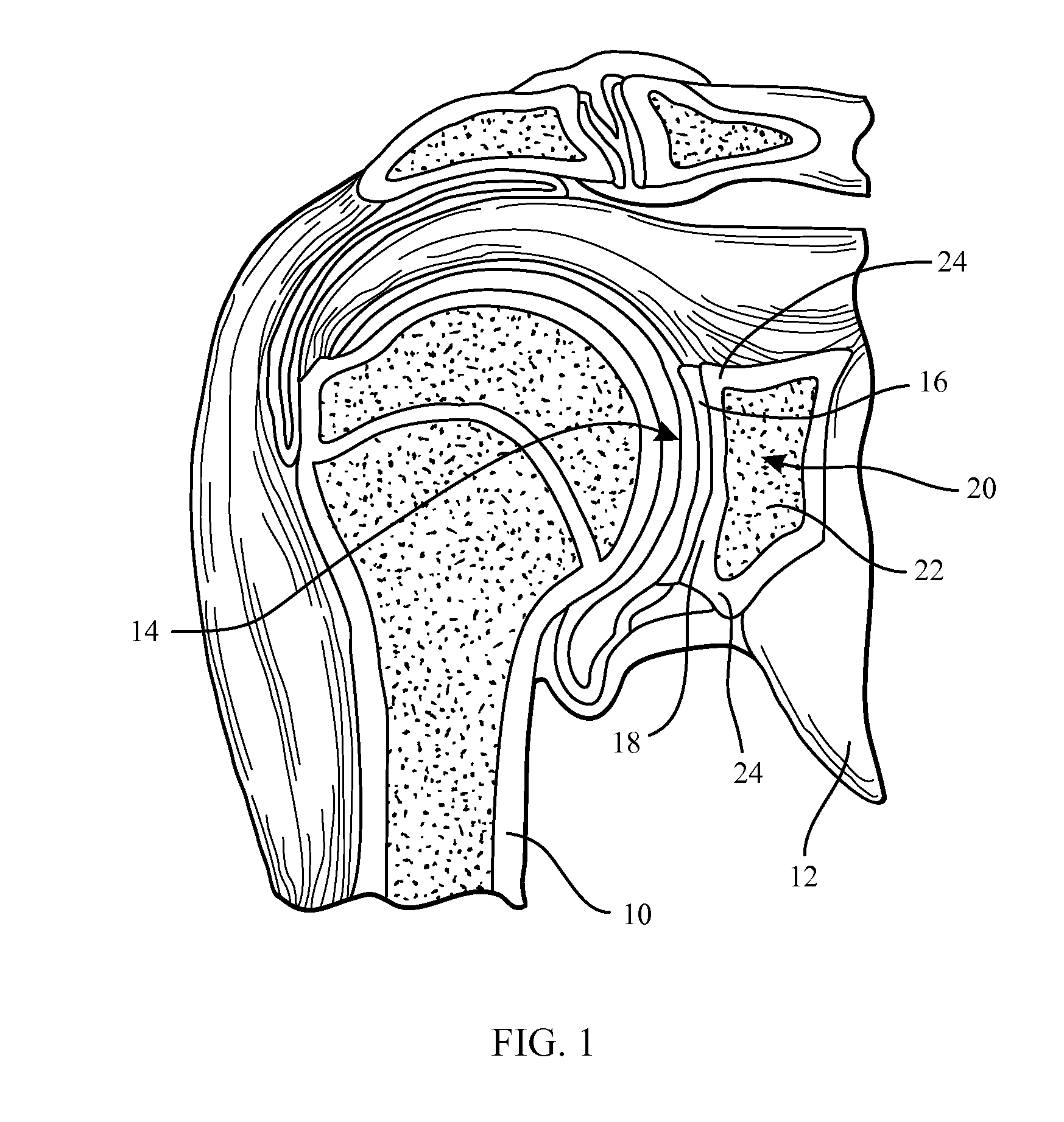
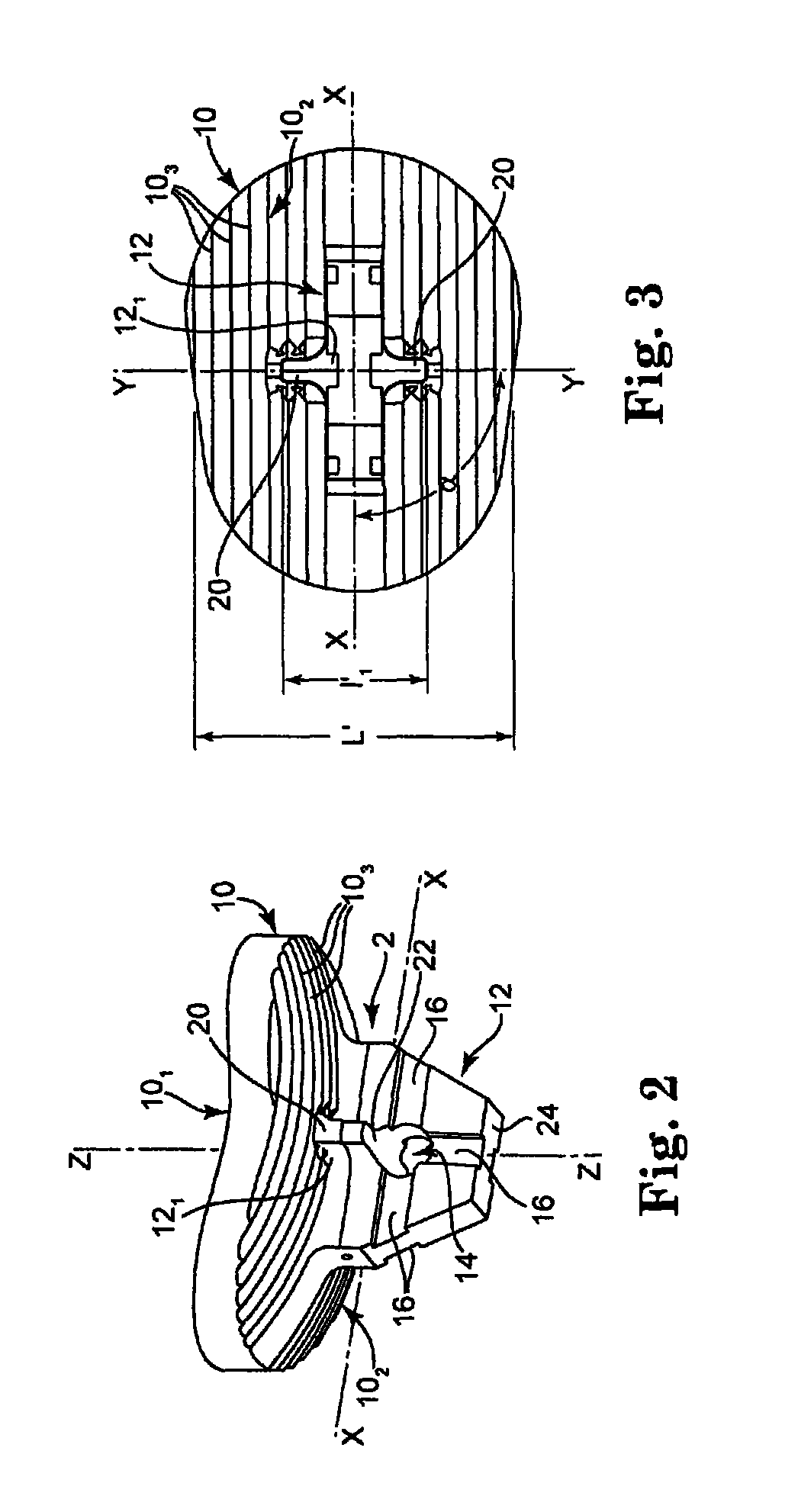
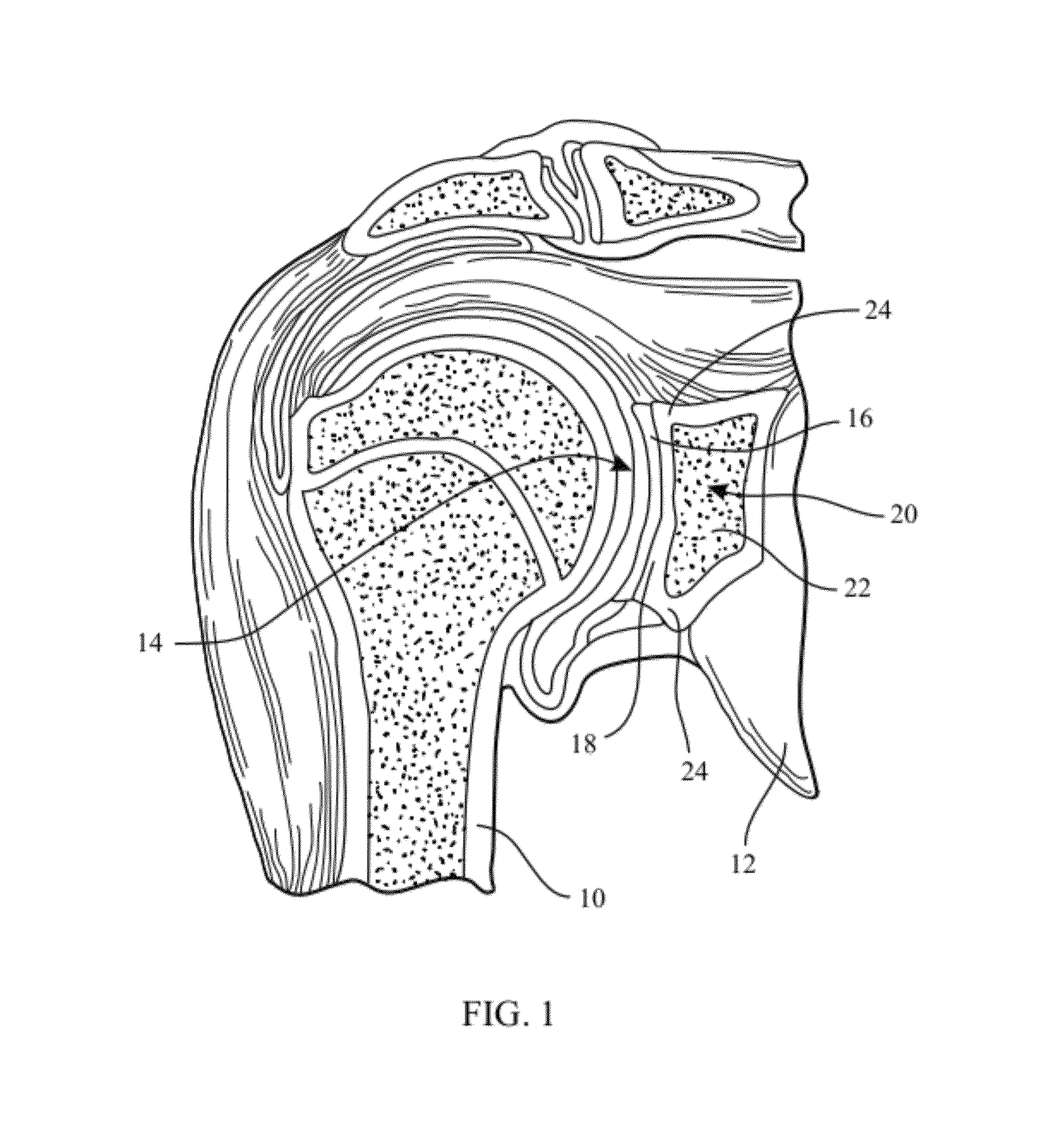
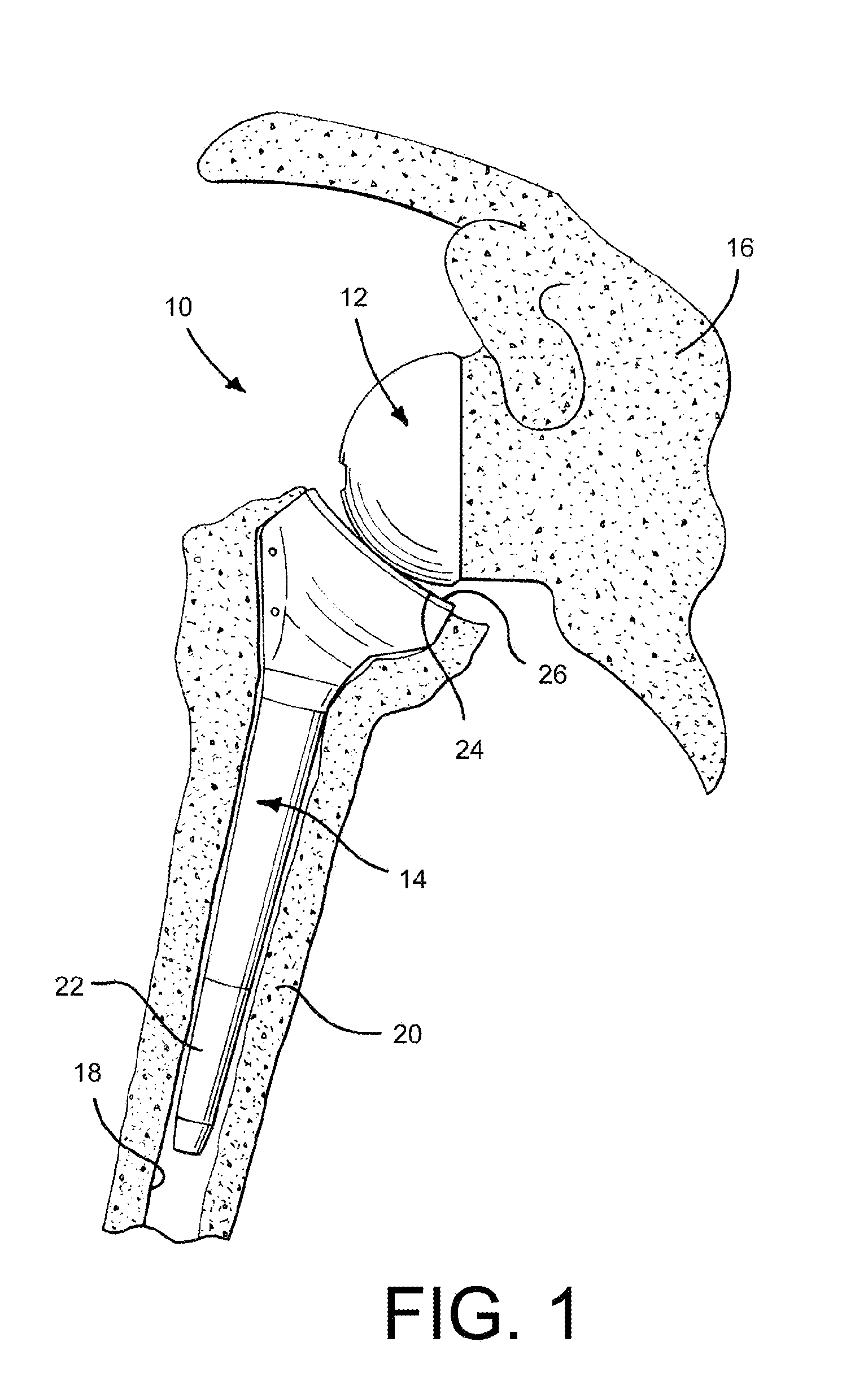
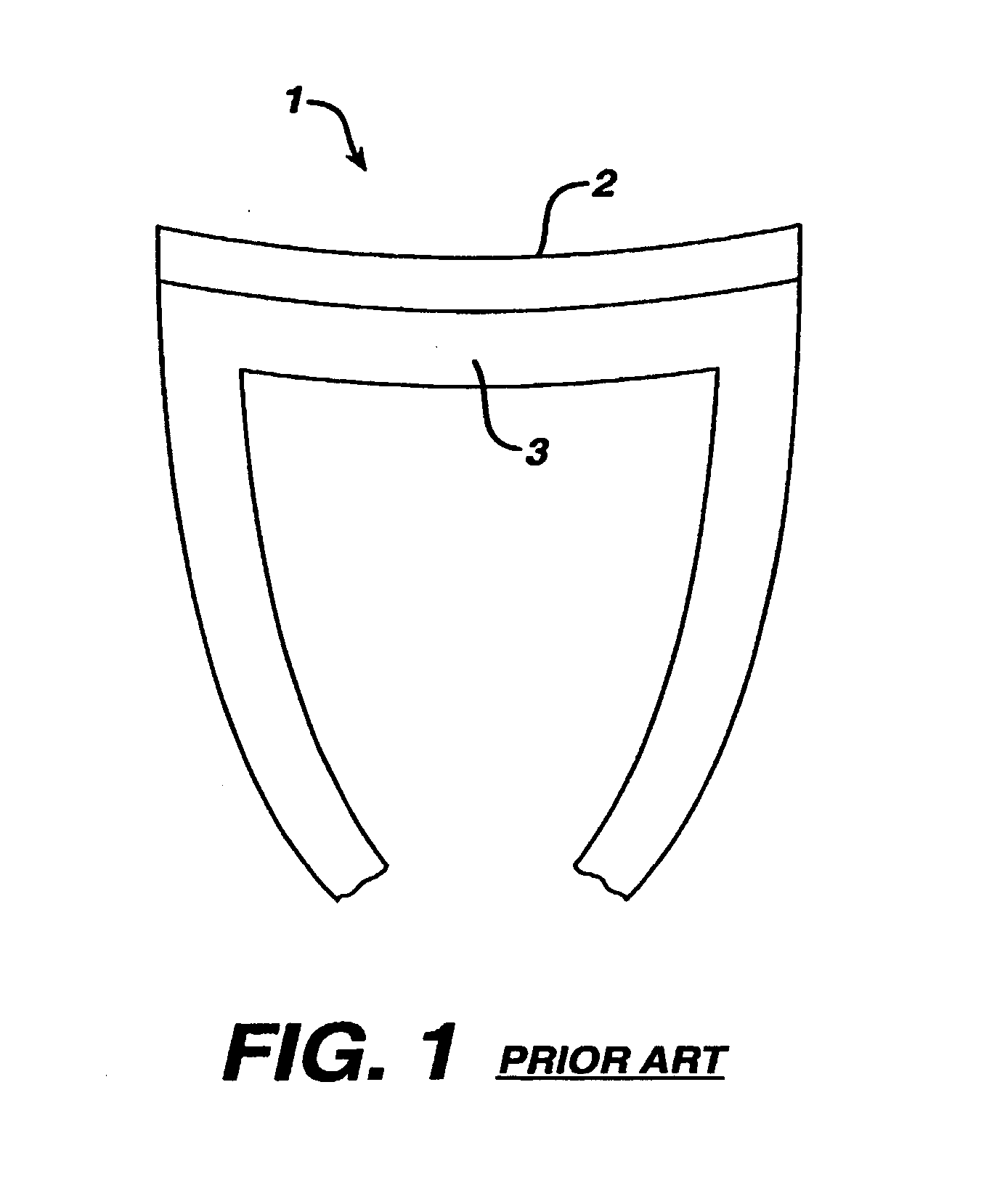
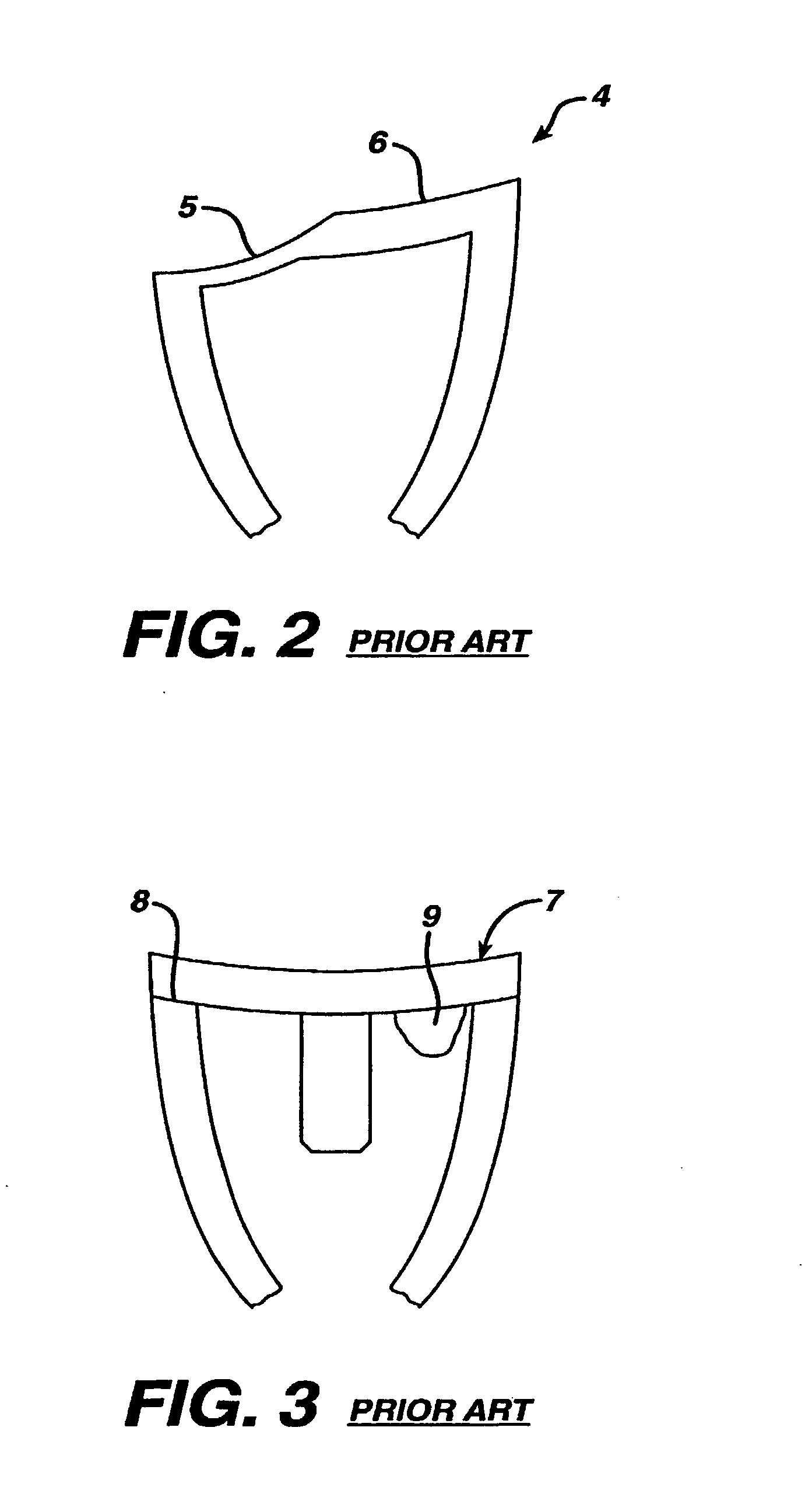
Glenoid Component. Historically, the glenoid component has been the “weak link” of shoulder replacement. In addition to being the most difficult part of the surgical procedure, the glenoid component is the most likely site of component failure, which has led many surgeons to avoid glenoid resurfacing in nearly all cases.
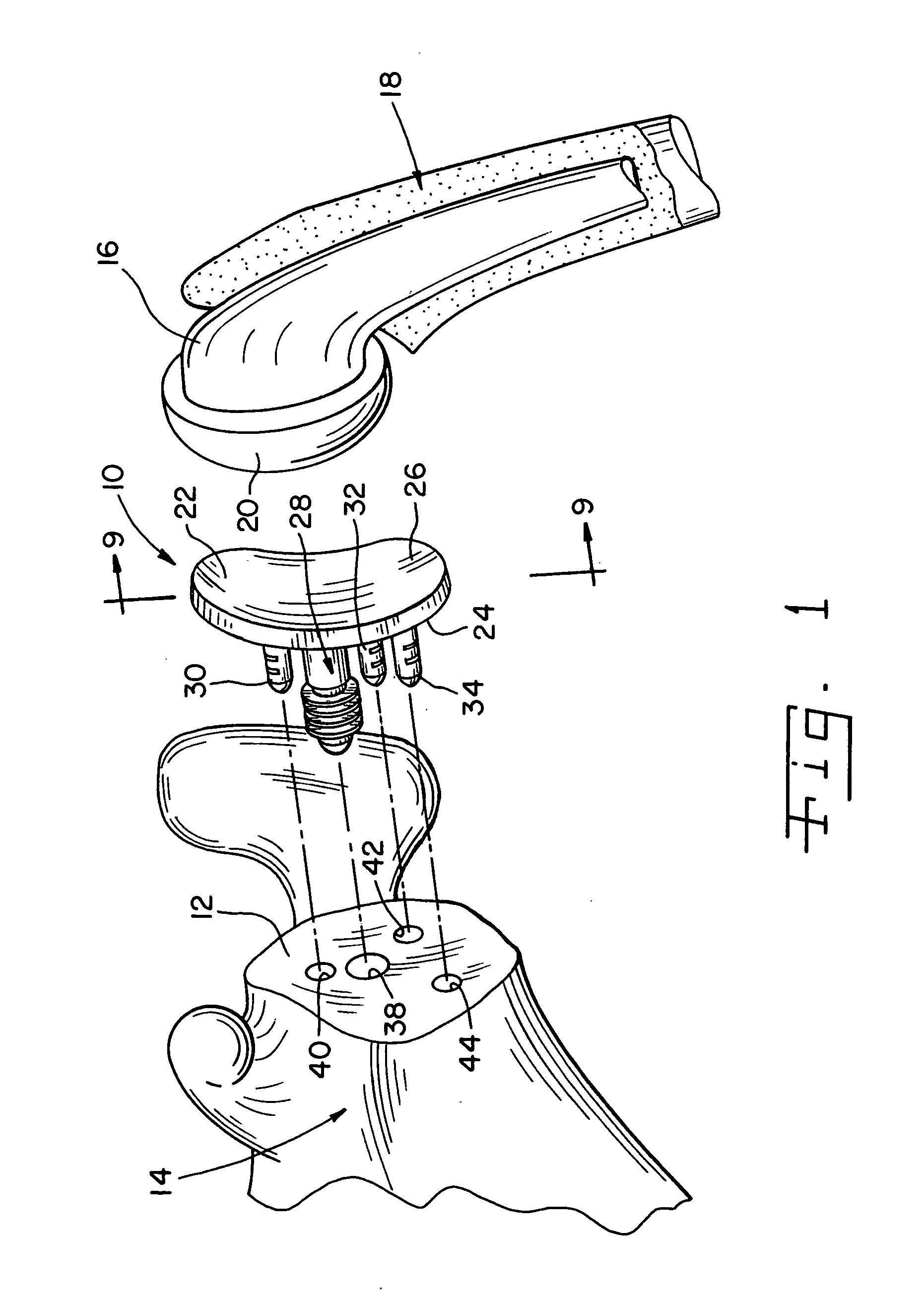
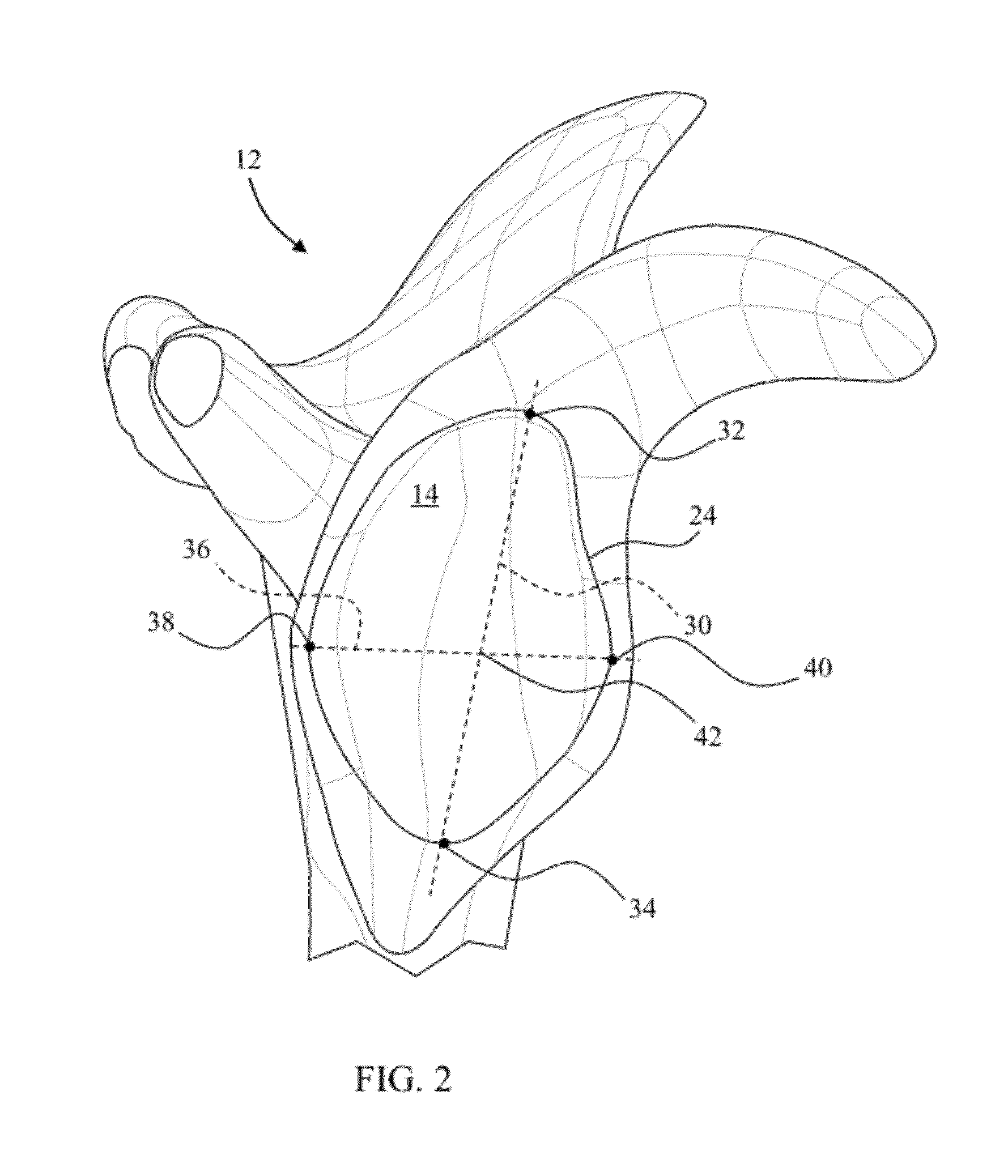
Glenoid alignment tool
An orthopaedic alignment guide component is provided for preparing a patient's bone socket, such as the glenoid of the patient's scapula, to receive a prosthetic glenoid component. The orthopaedic alignment guide component includes a guide body for inserting a guide pin into the patient's scapula and an inferior referencing arm for referencing an inferior surface of the patient's scapula.
Owner:ZIMMER GMBH
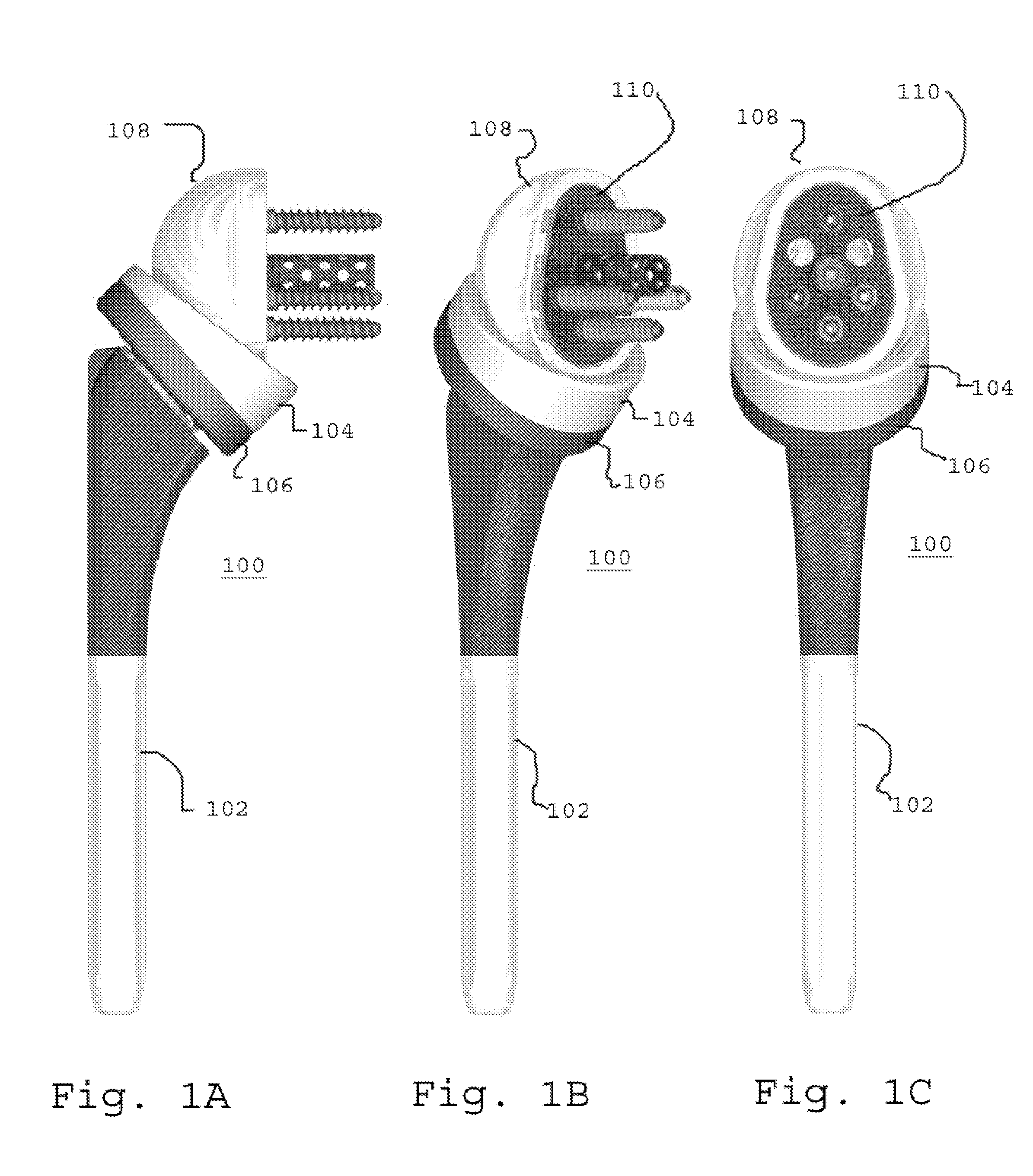
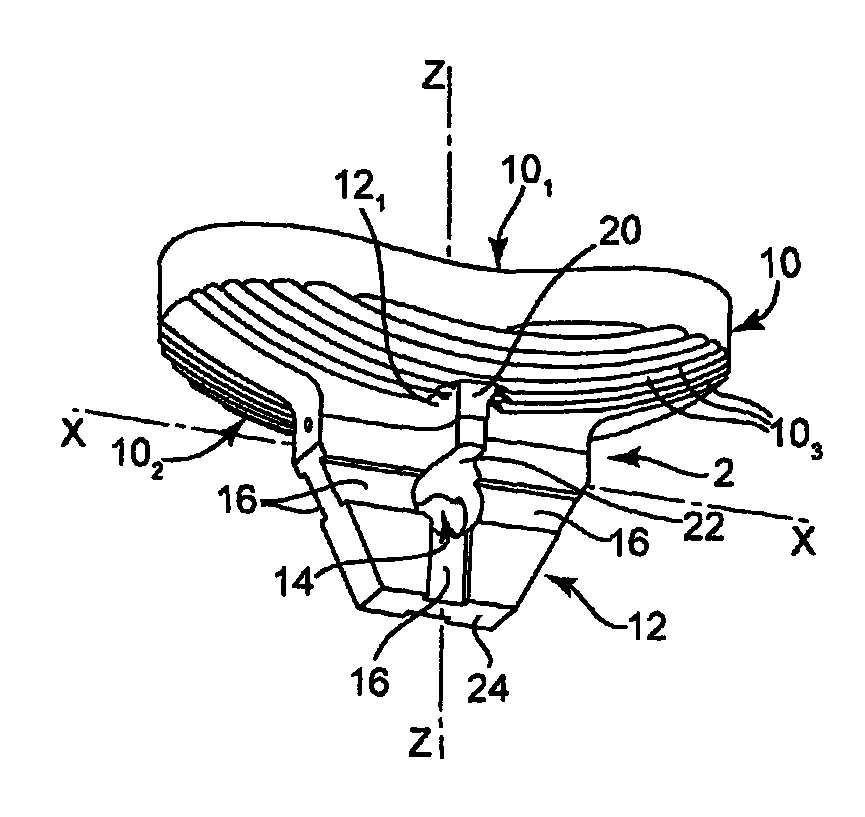
Glenoid component of a shoulder prosthesis and complete shoulder prosthesis incorporating such a component
The glenoid component according to the invention comprises a metal body of which the inner face is adapted to be immobilized on the glenoid cavity of a shoulder and of which the outer face bears a concave articulating surface adapted to cooperate with a humeral component. This articulating surface extends on the periphery by a convex surface forming, at least in part, the edge of the body.
Owner:TORNIER SA SAINT ISMIER
Glenoid component for shoulder arthroplasty
ActiveUS20080140209A1Additive manufacturing apparatusJoint implantsShoulder hemiarthroplastySacroiliac joint
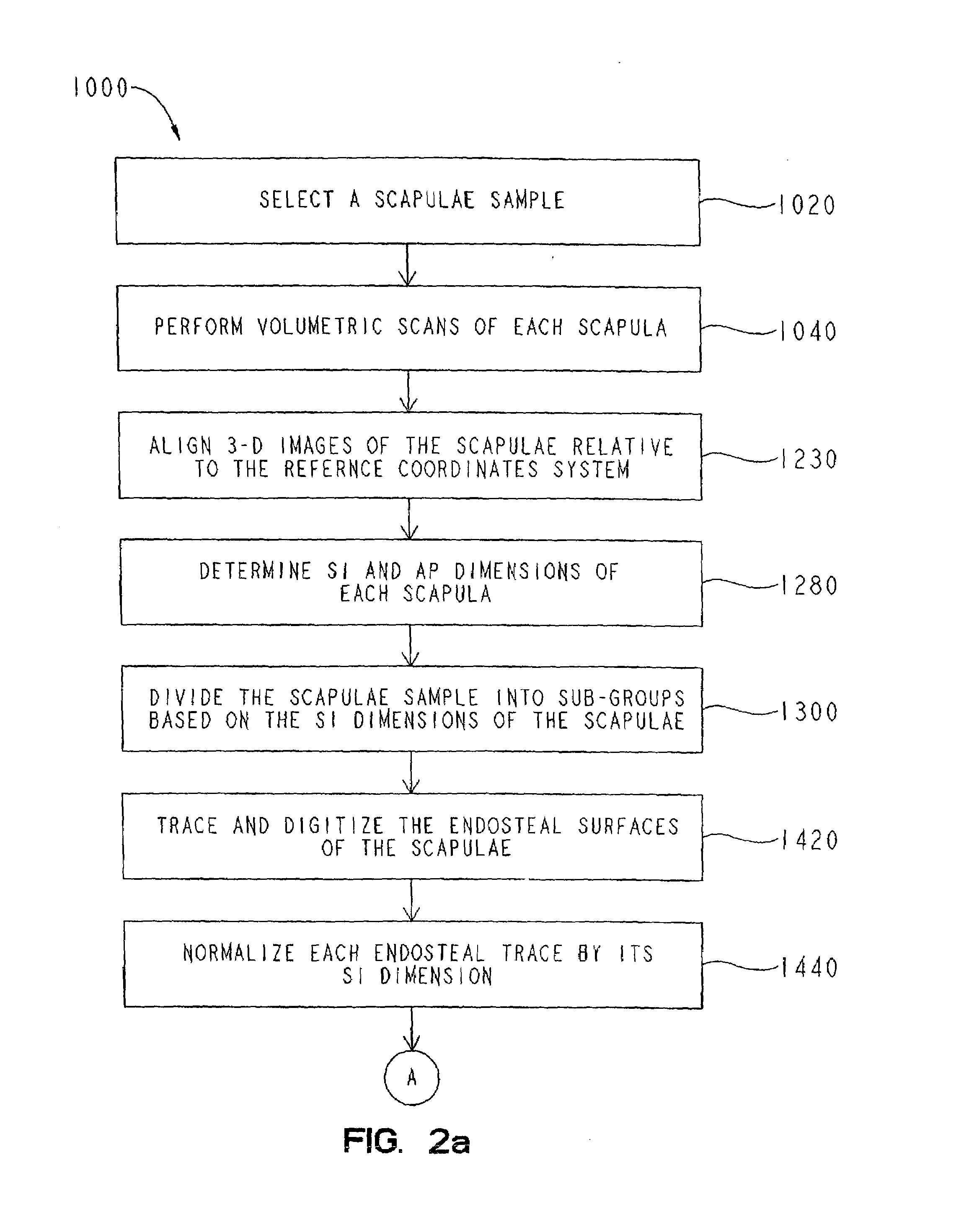
A glenoid component apparatus for shoulder arthroplasty includes a bearing portion and a stem portion connected to the bearing portion. The stem portion is modeled from a normalized glenoid vault morphology. A method for making a glenoid component for shoulder arthroplasty includes obtaining a model of a normalized glenoid vault morphology and producing a stem portion of the glenoid component based on the model.
Owner:DEPUY PROD INC
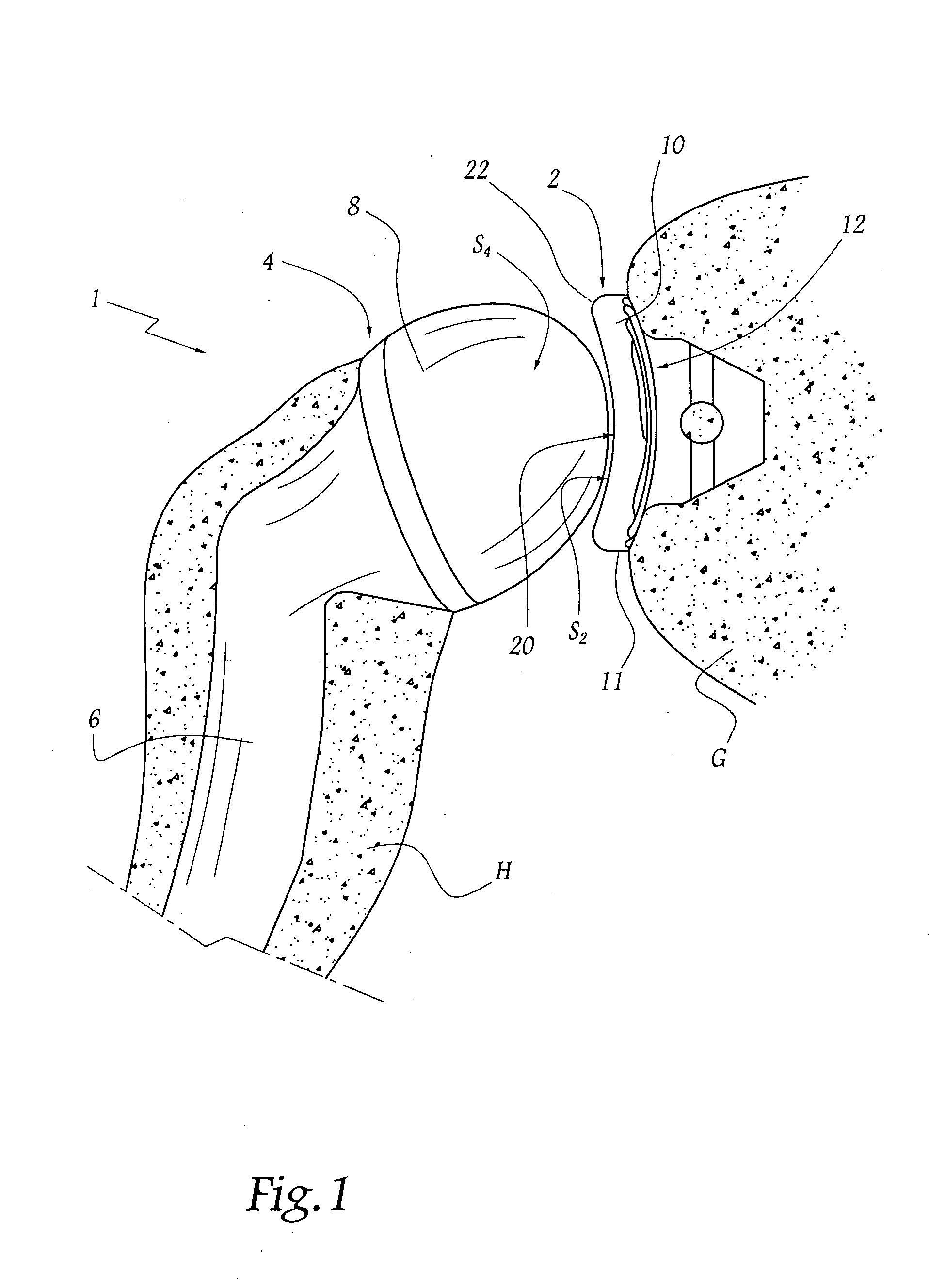
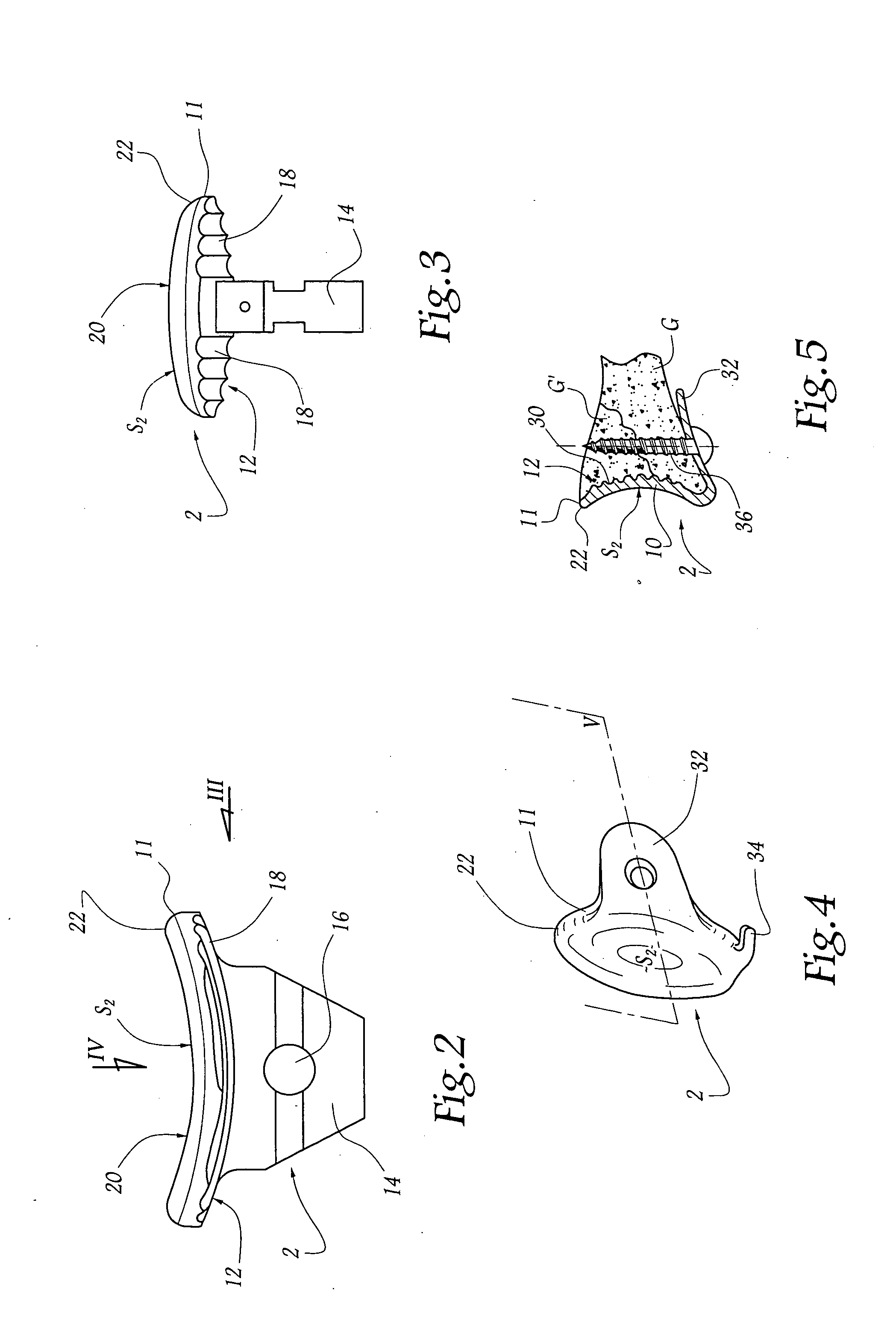
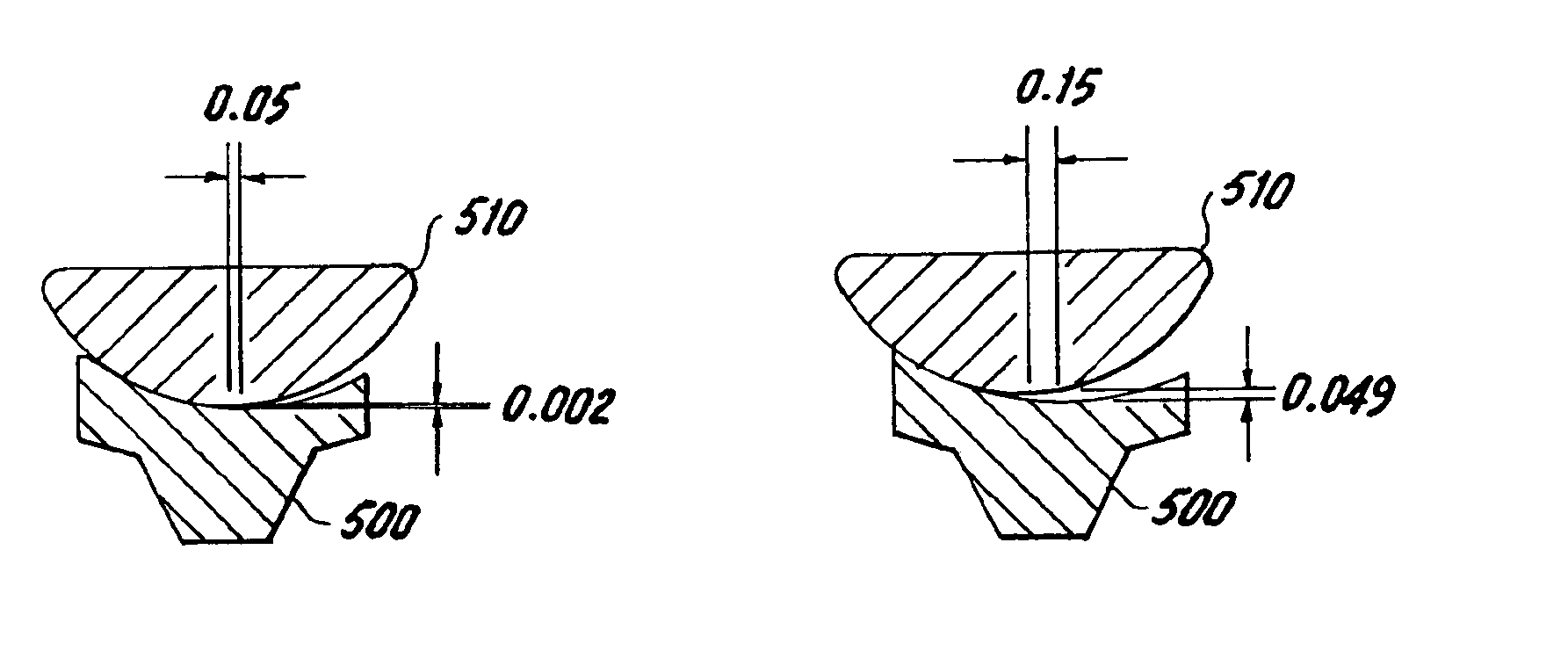
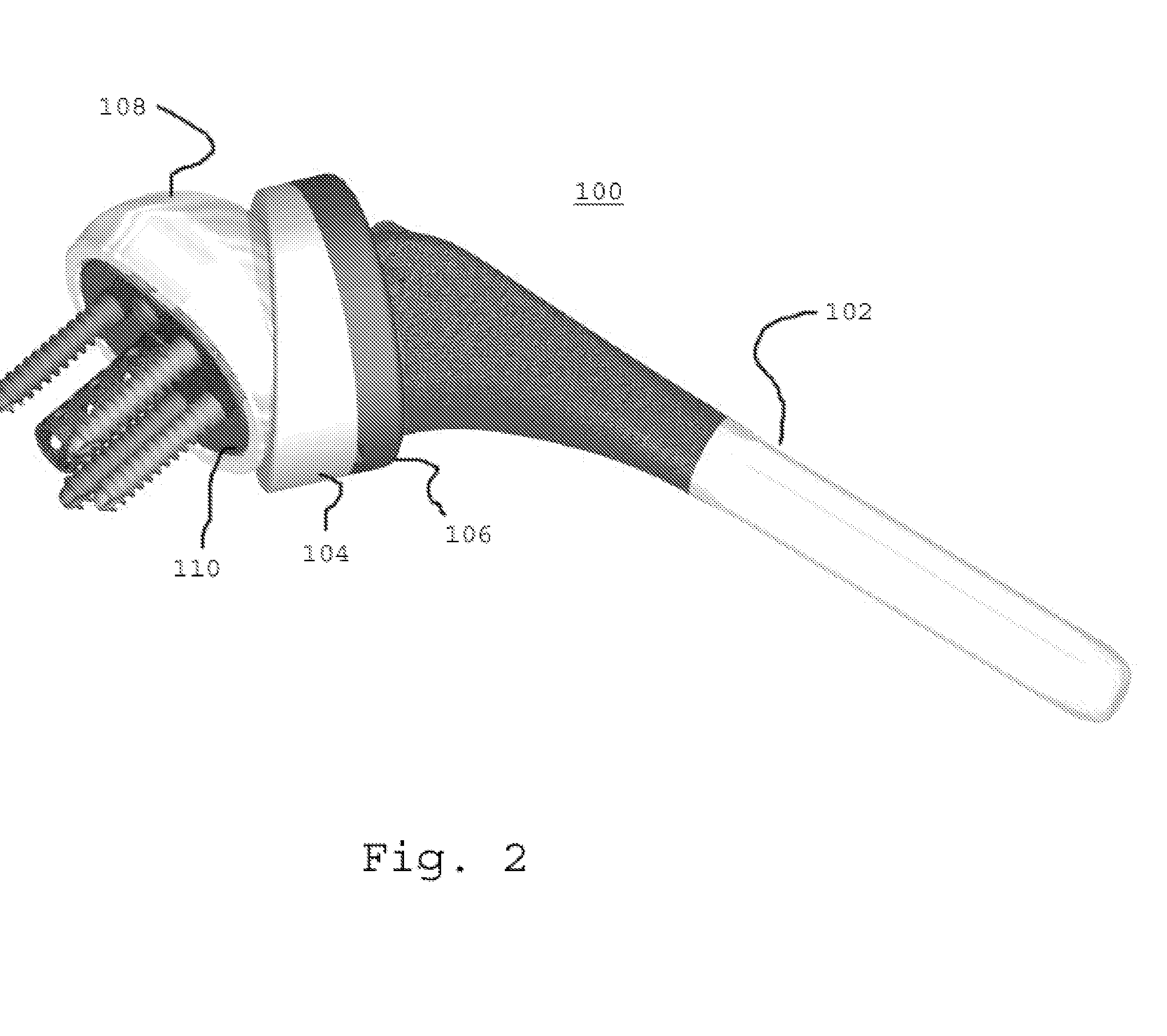
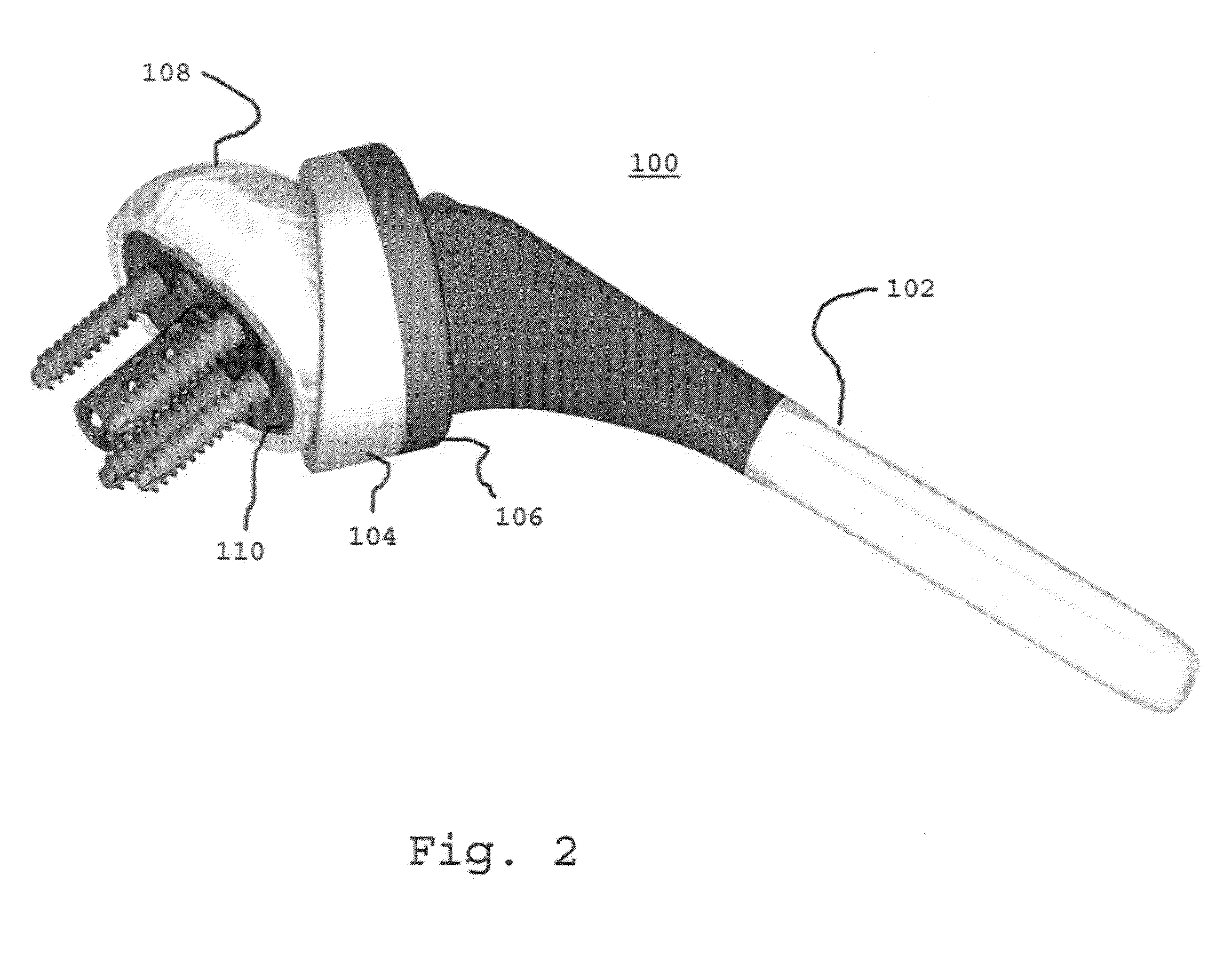
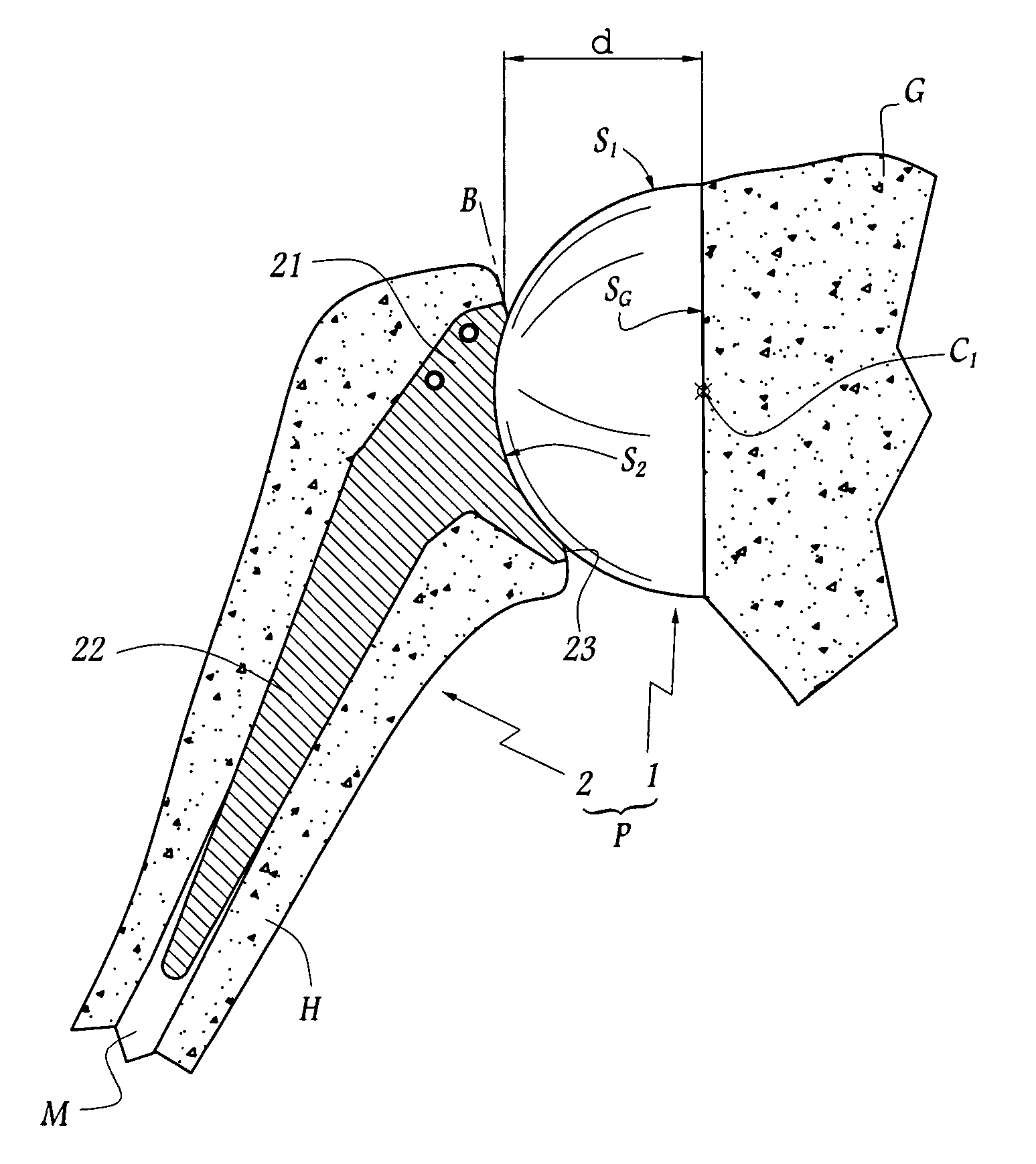
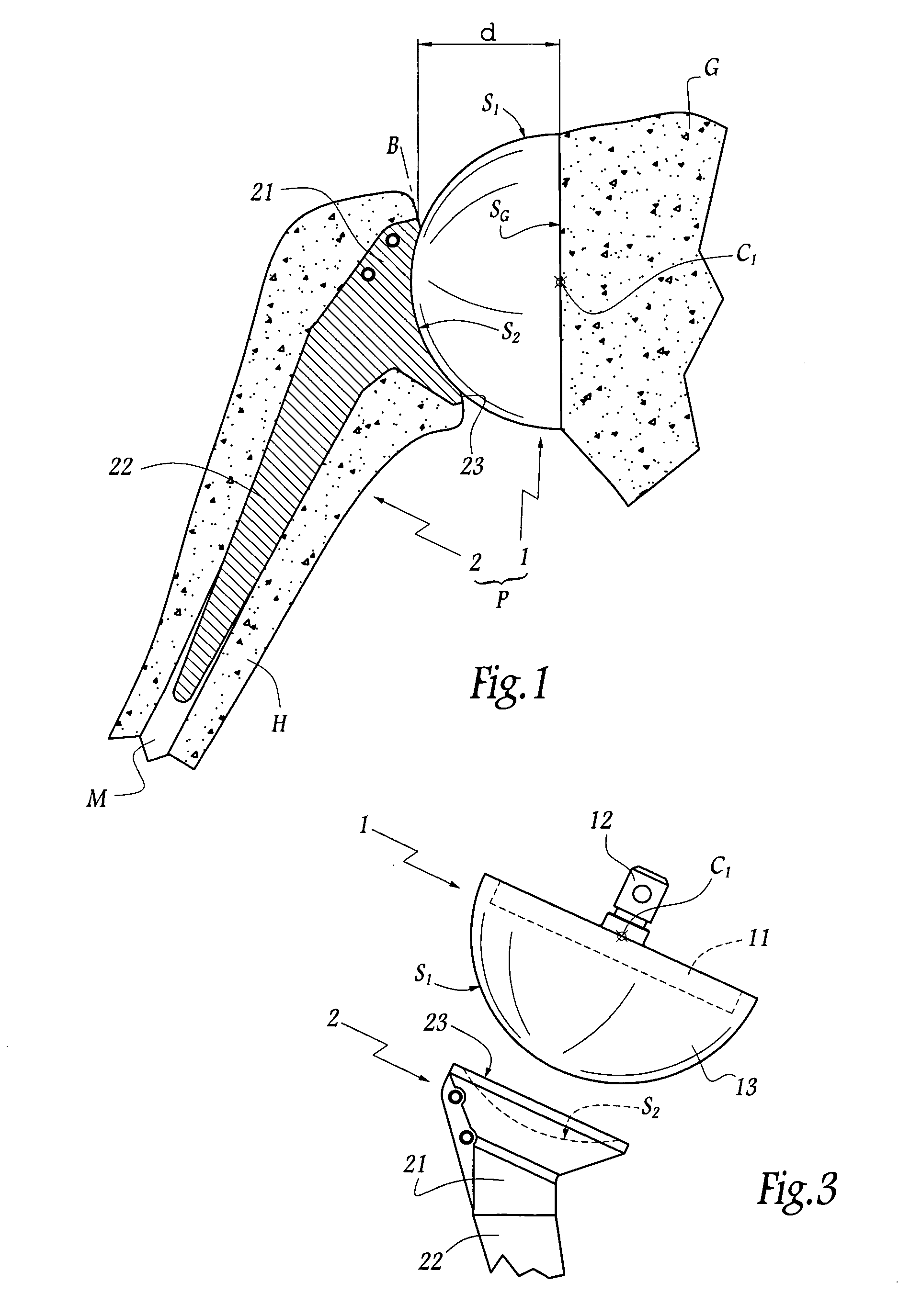
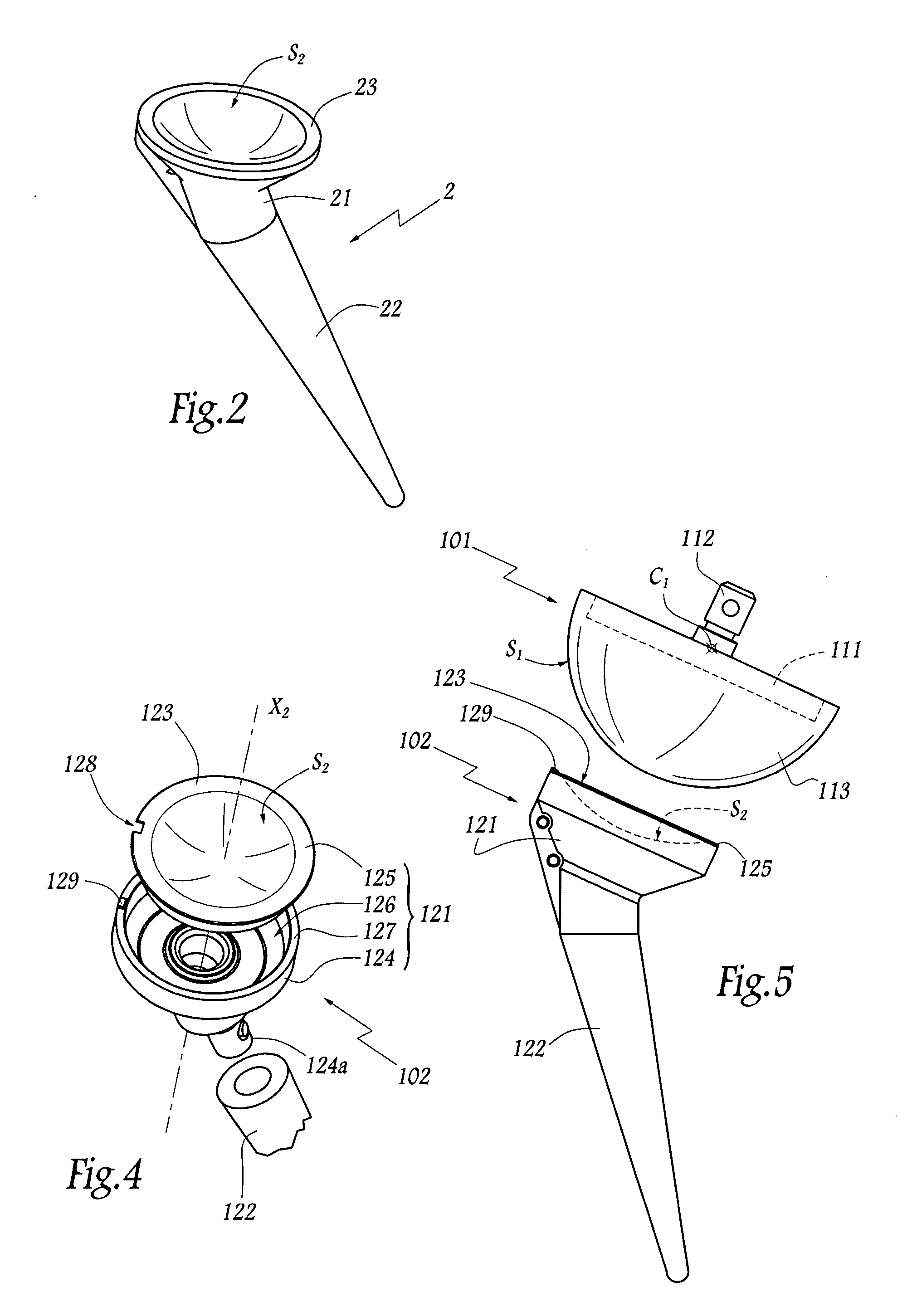
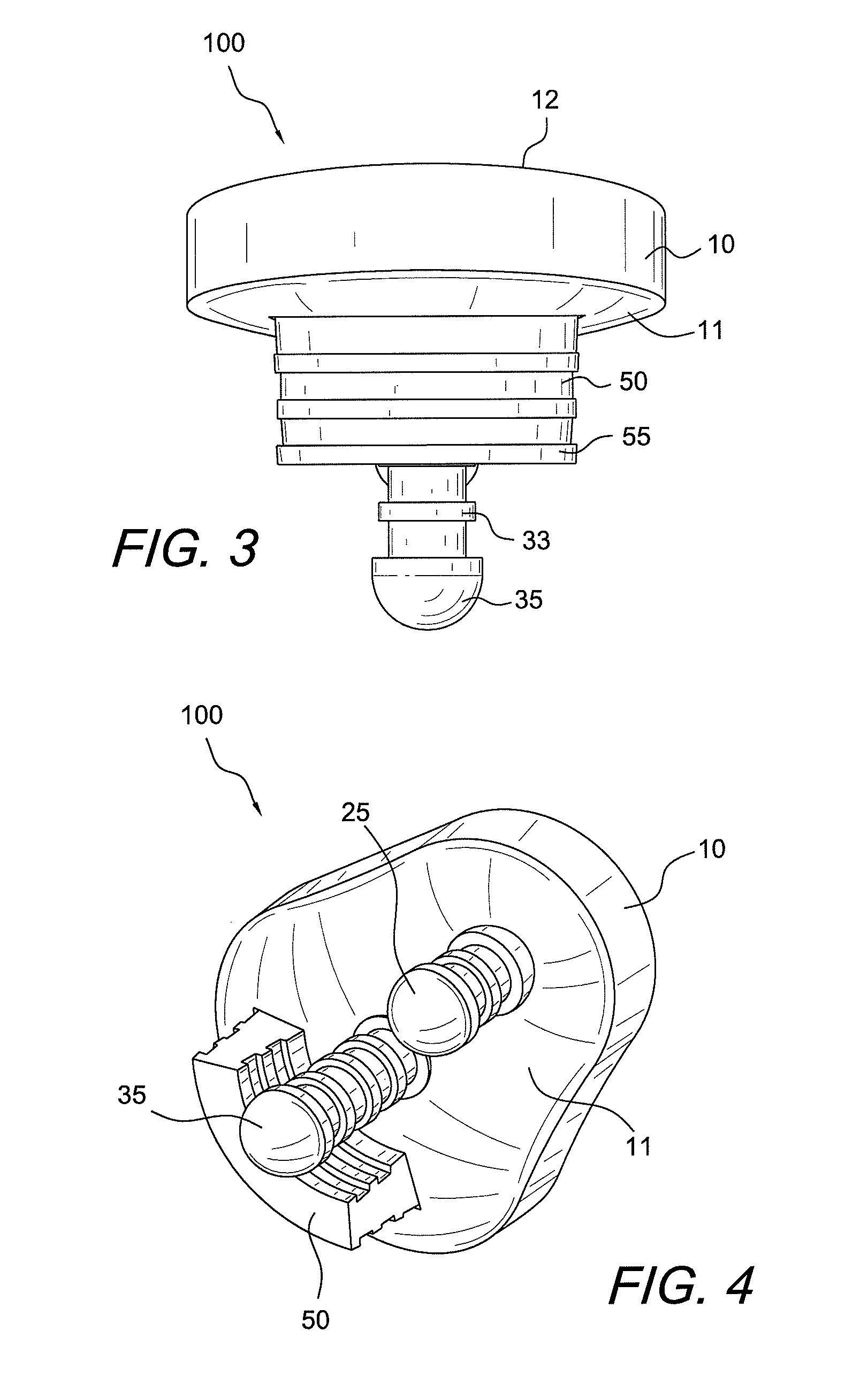
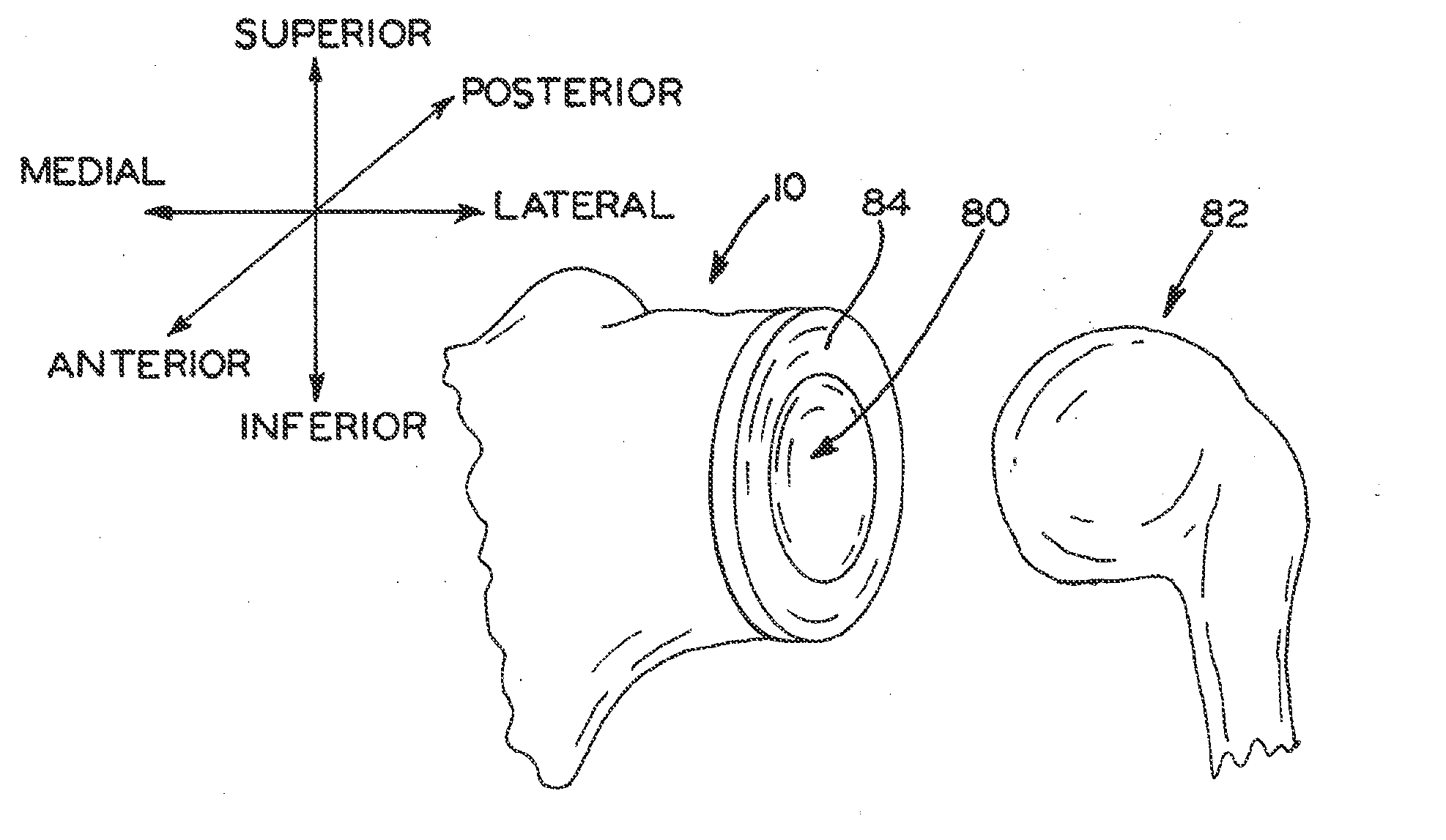
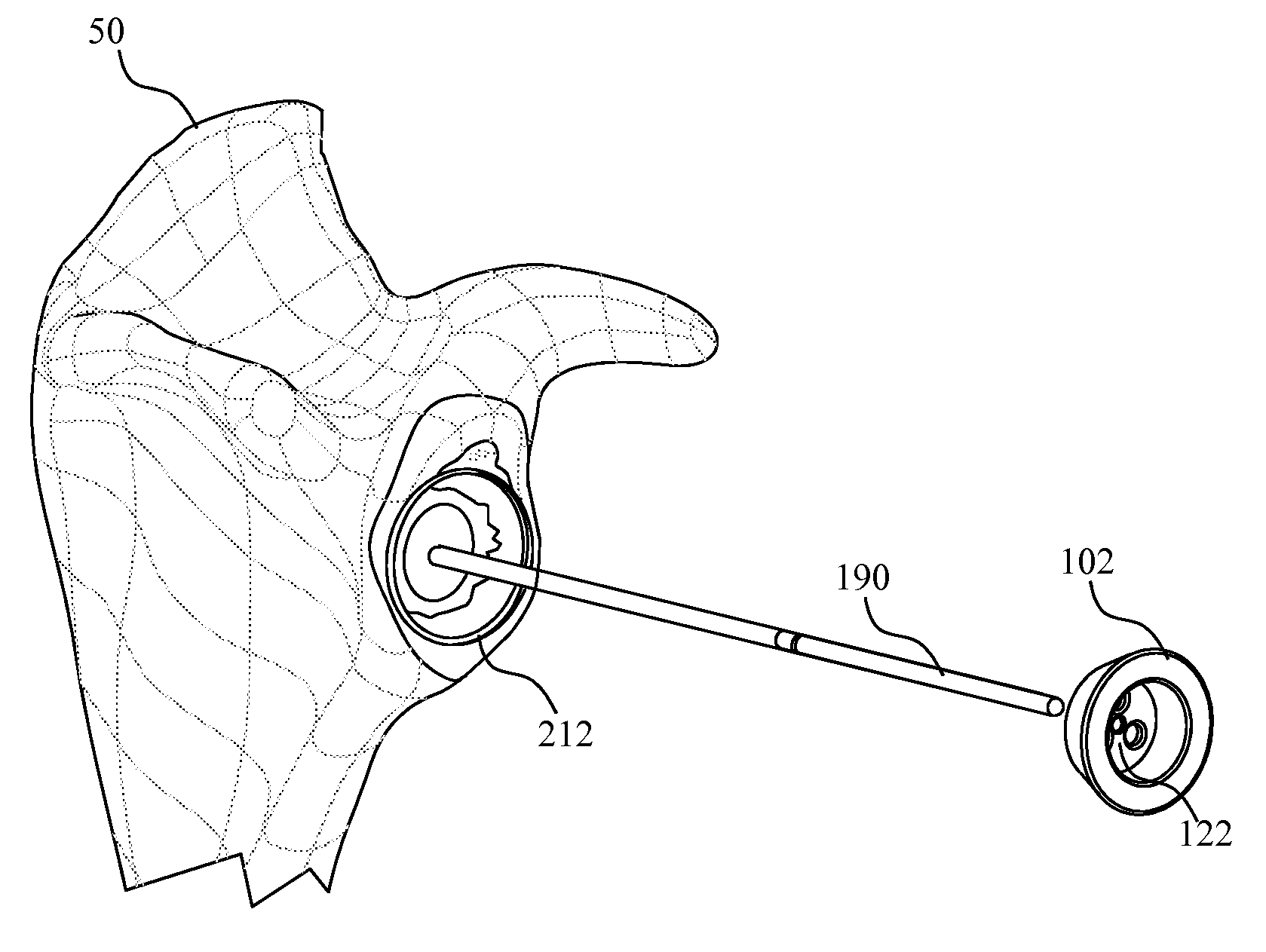
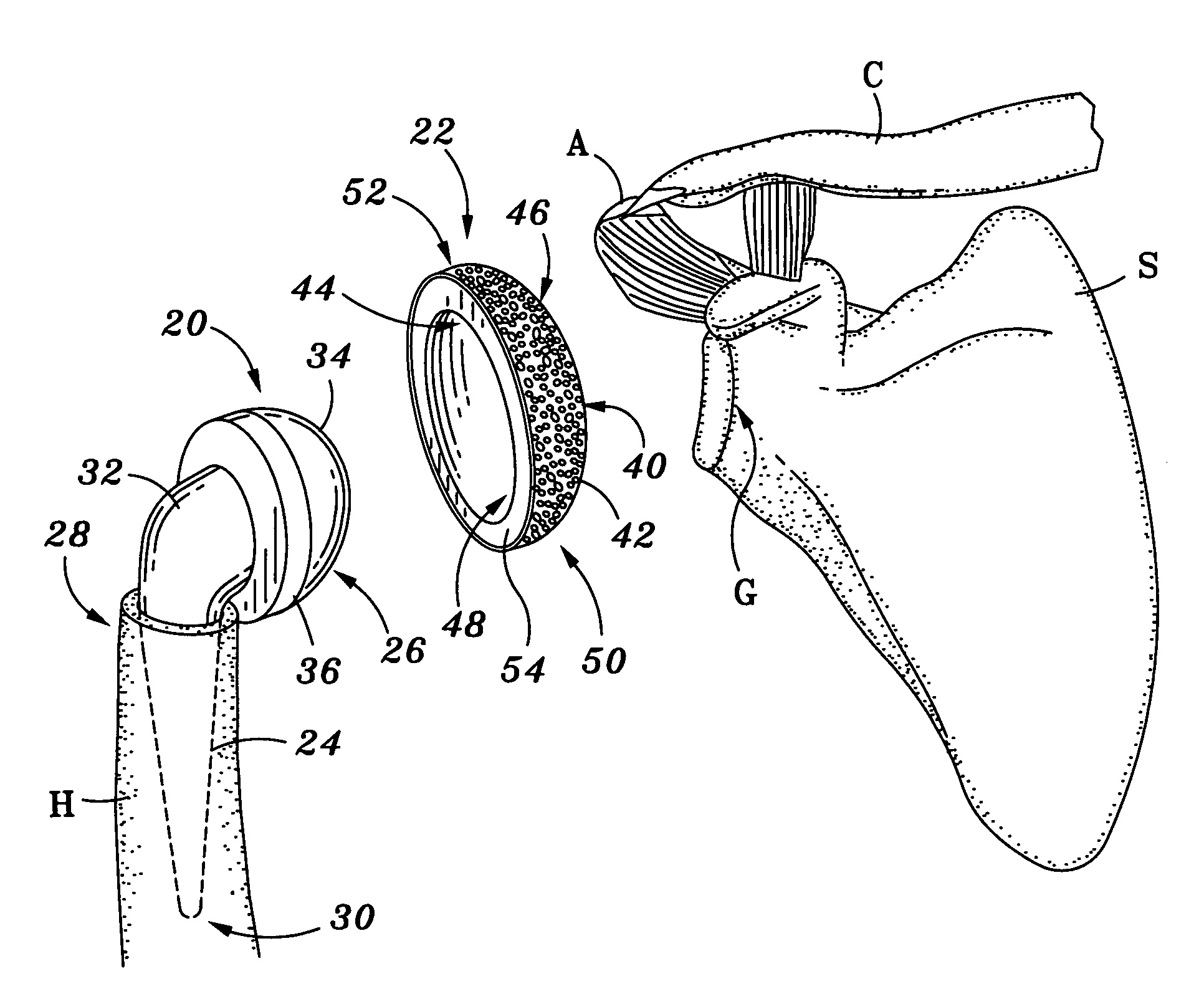
Concave resurfacing prosthesis
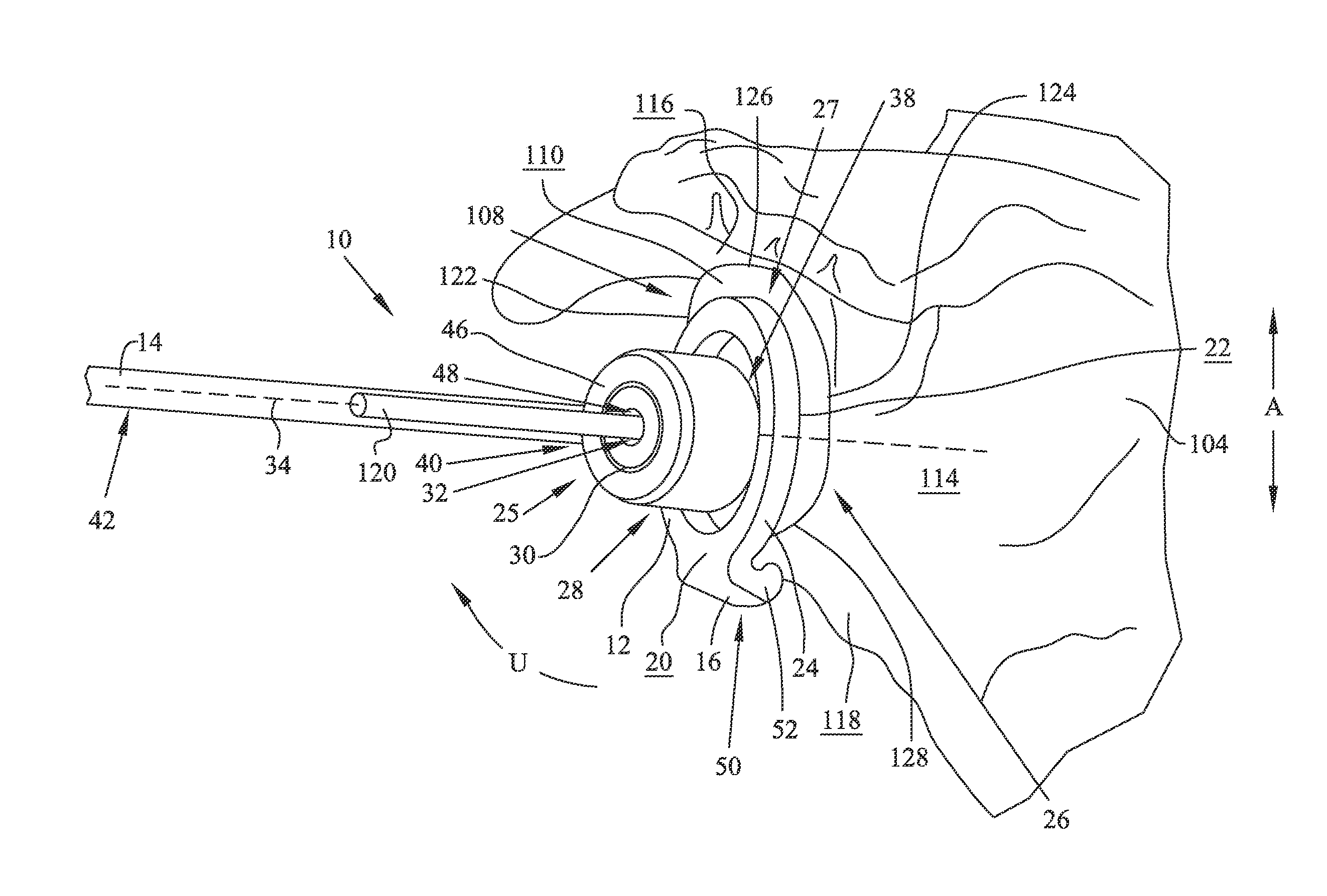
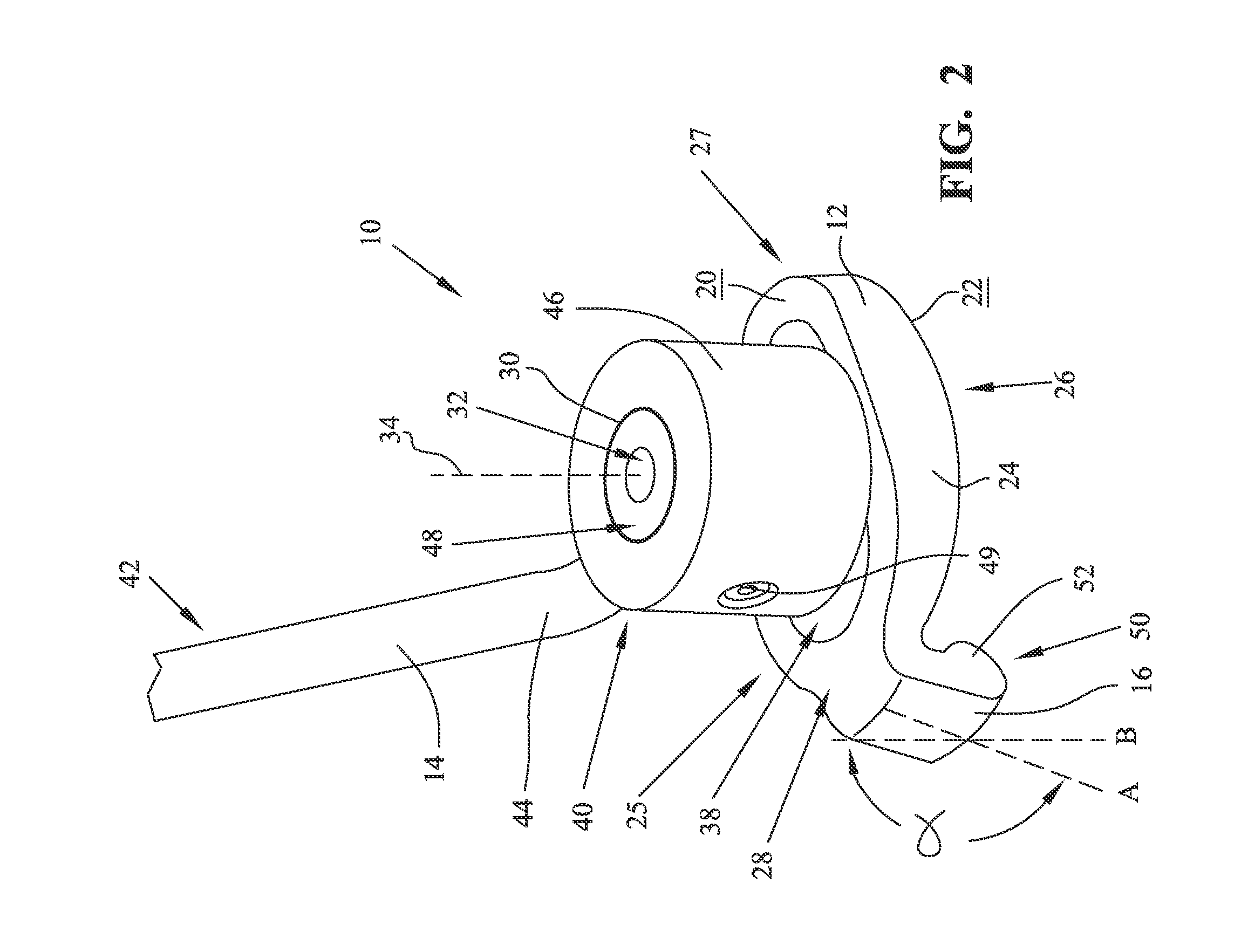
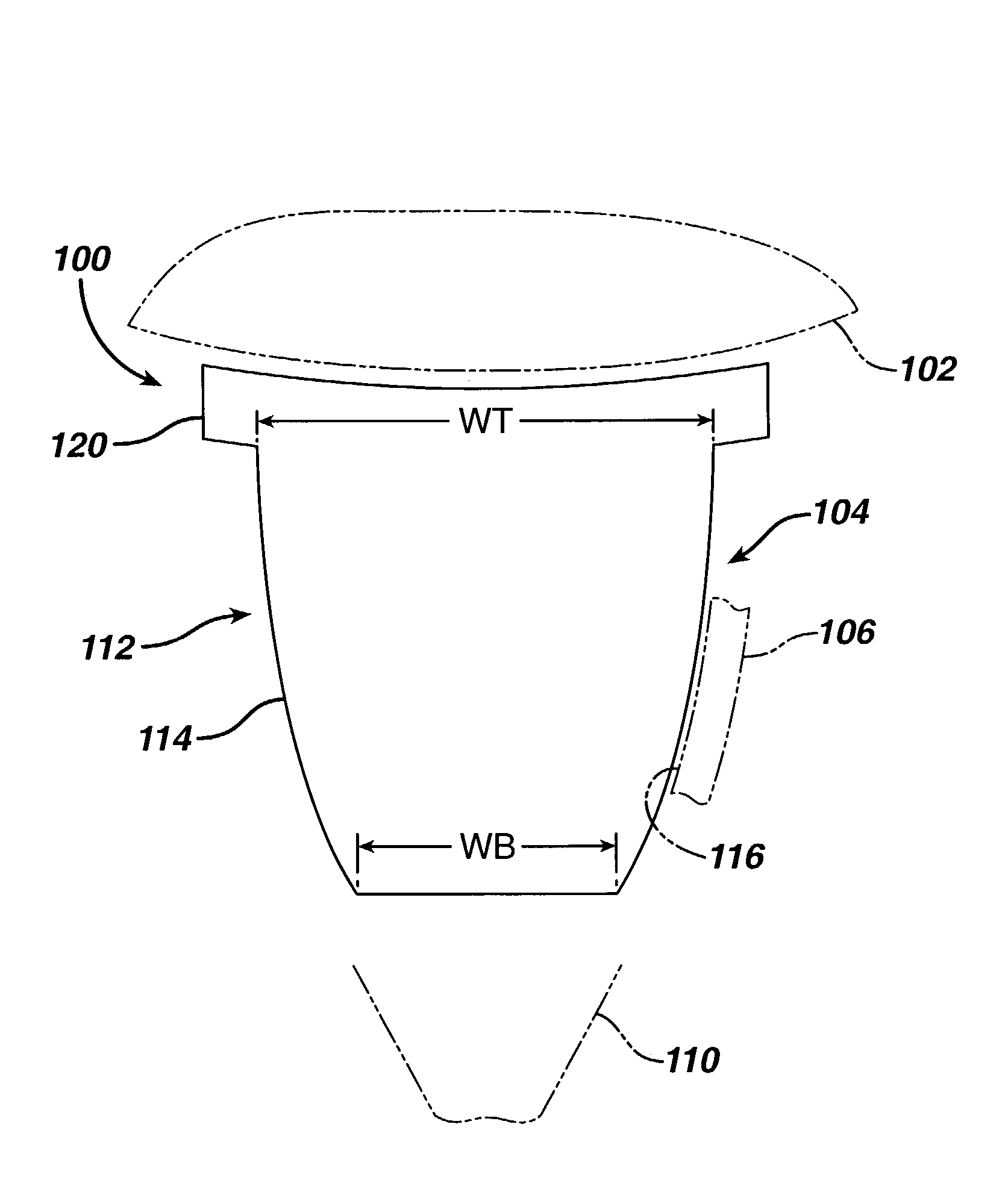
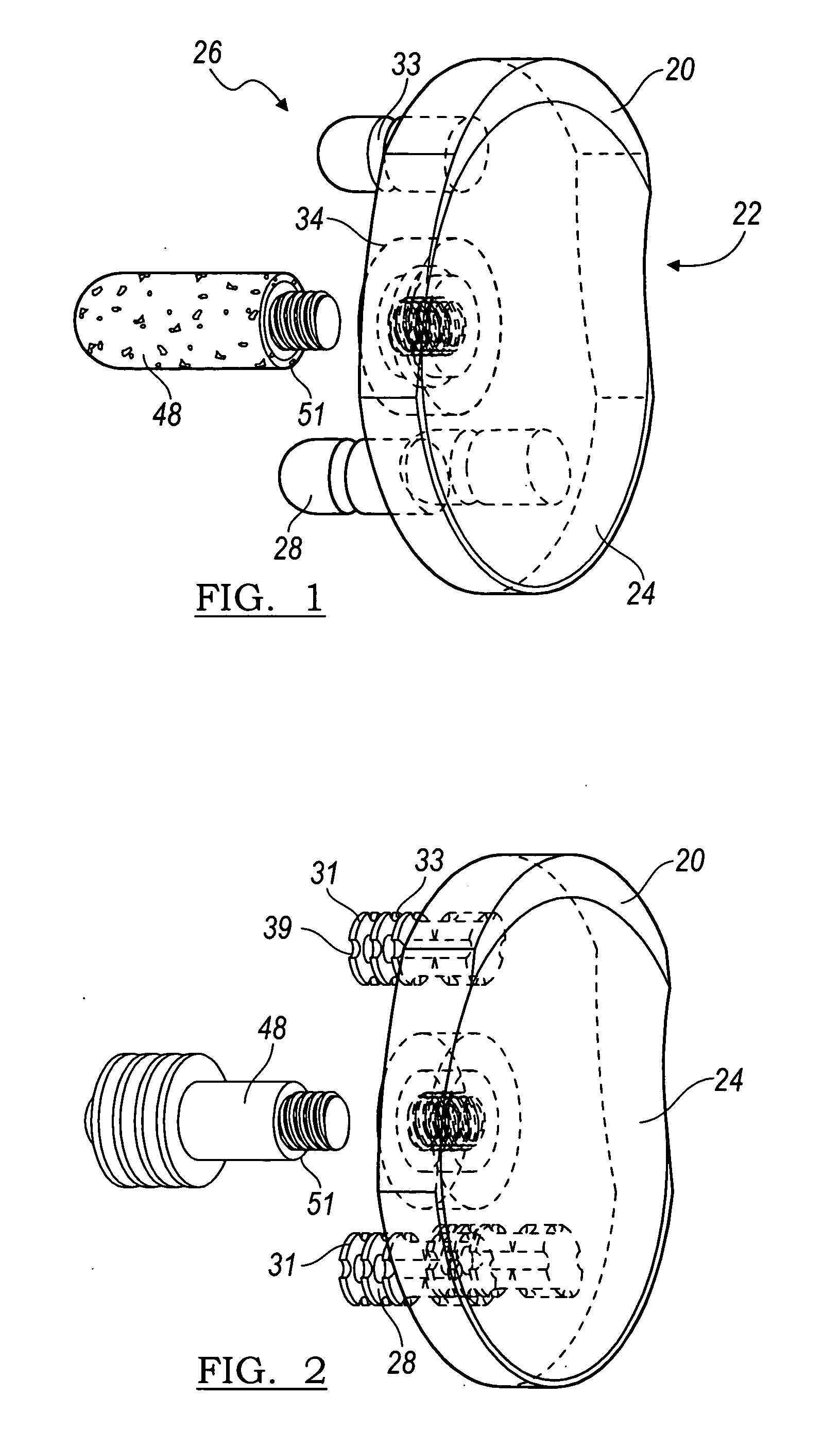
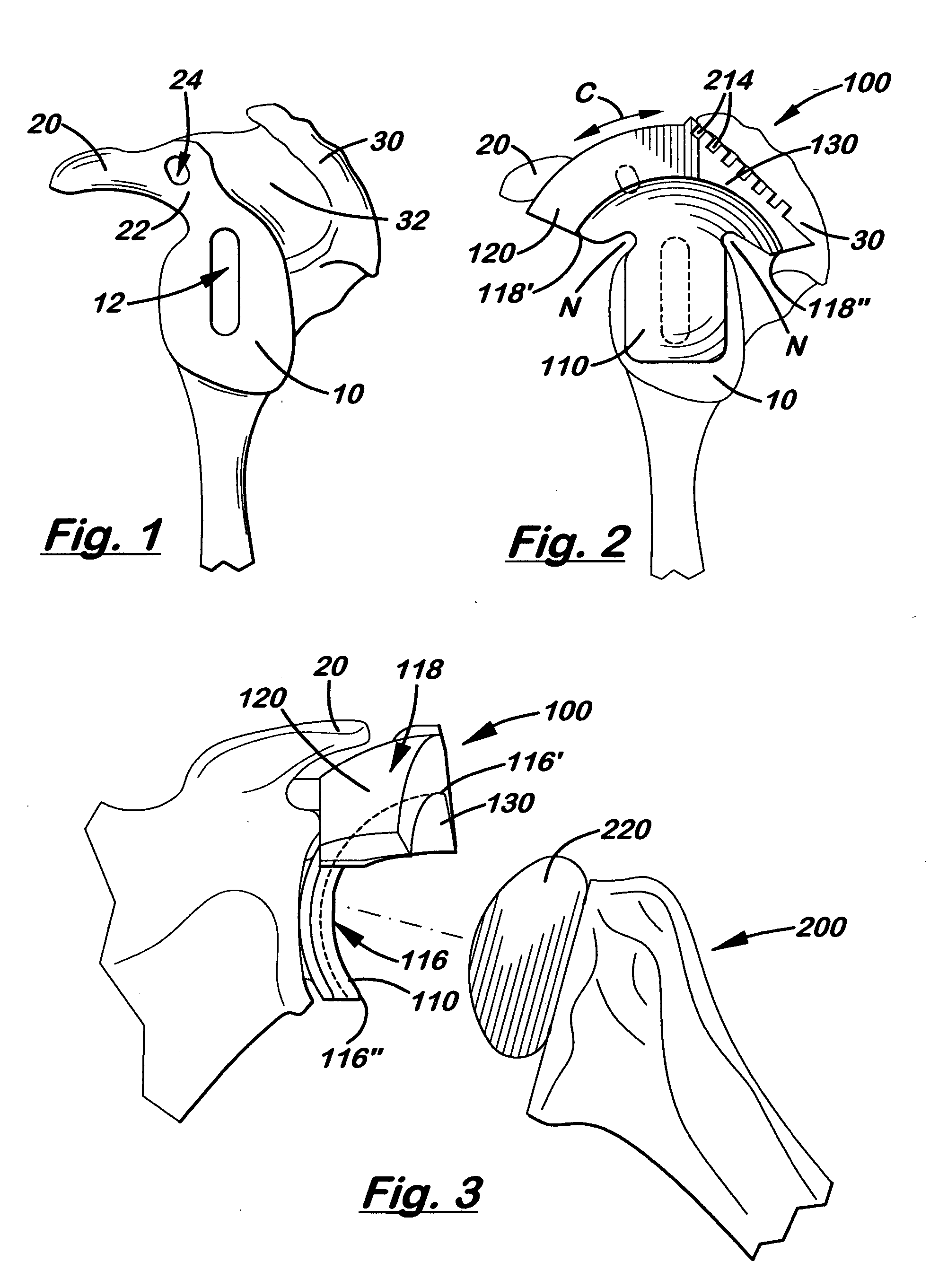
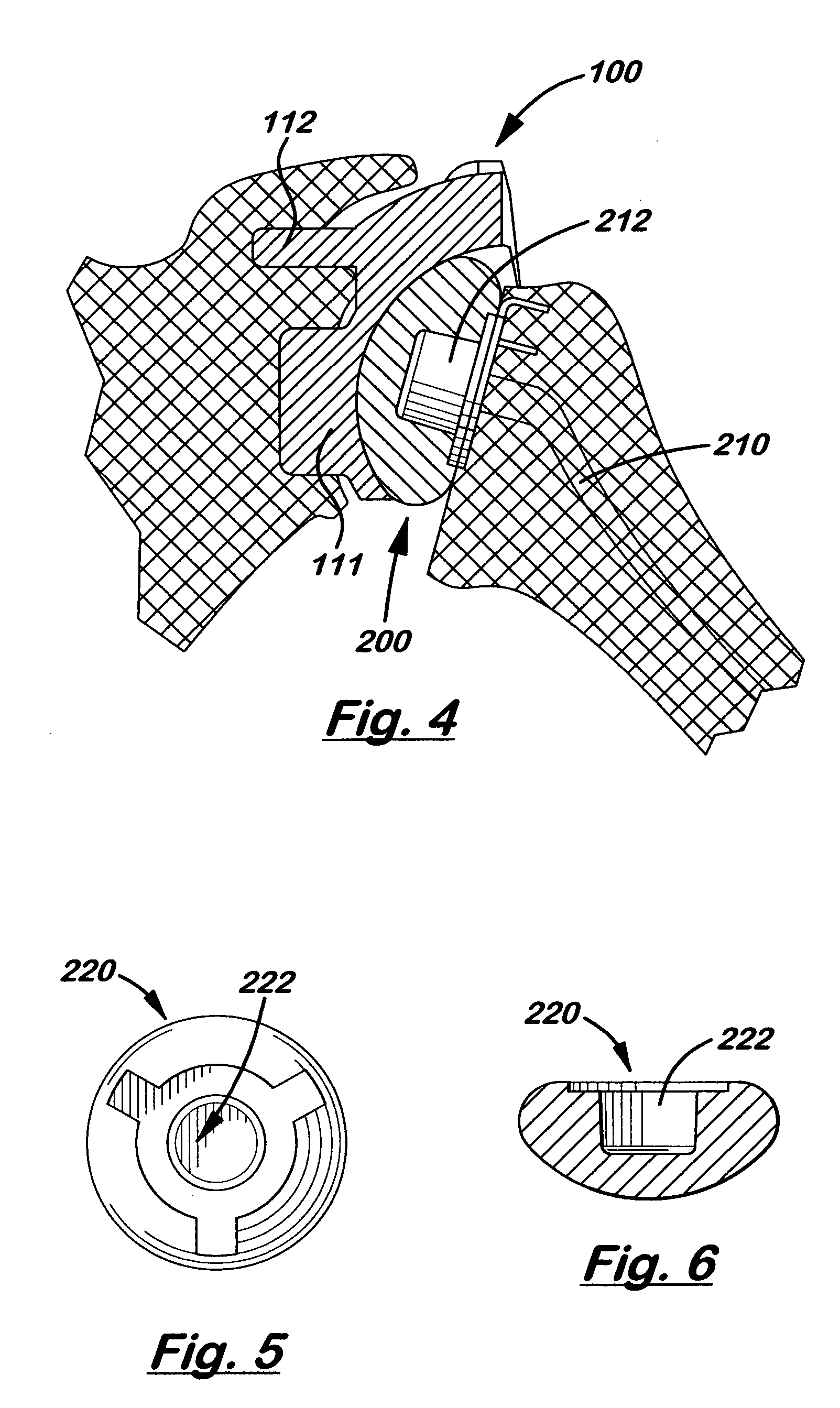
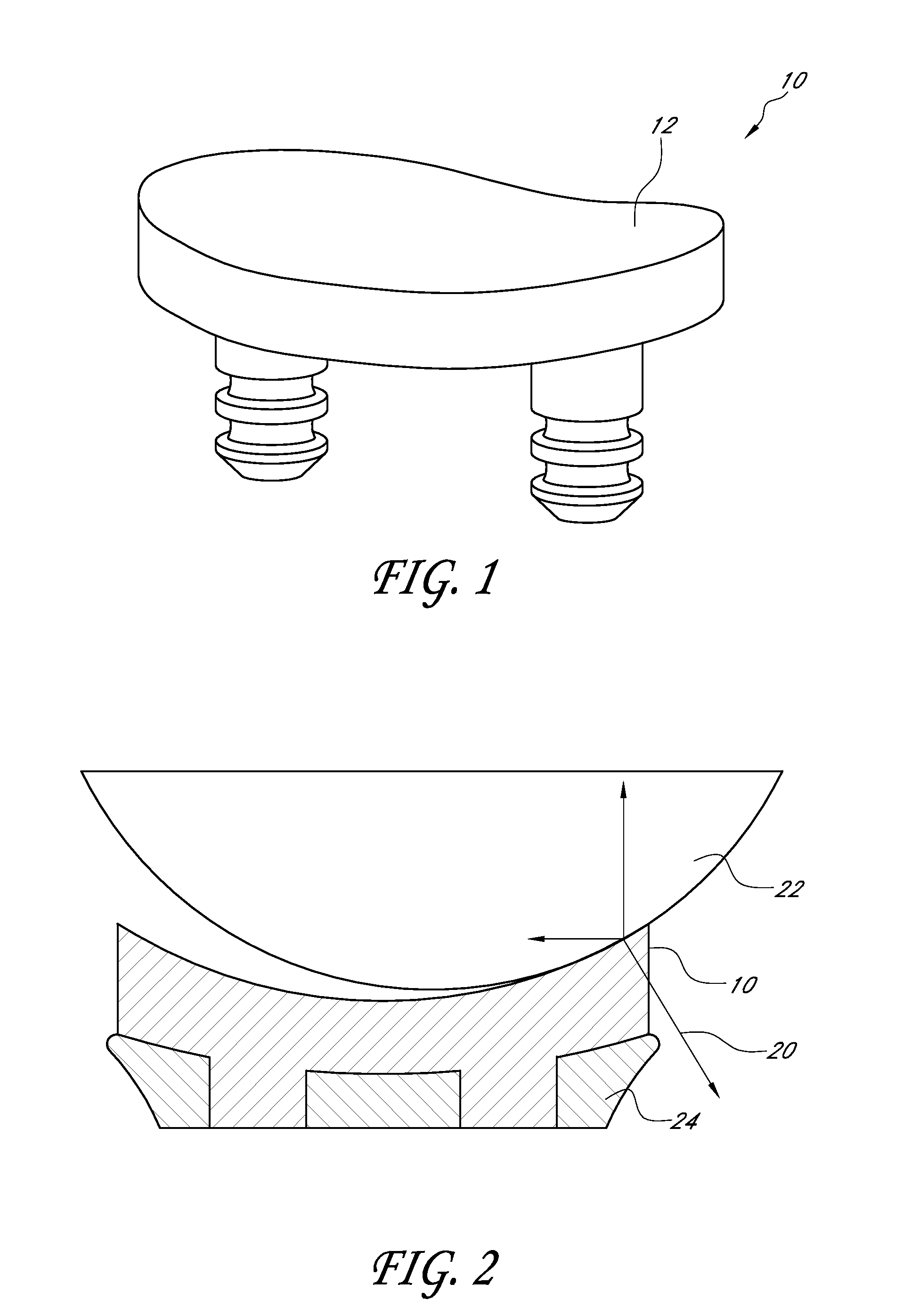
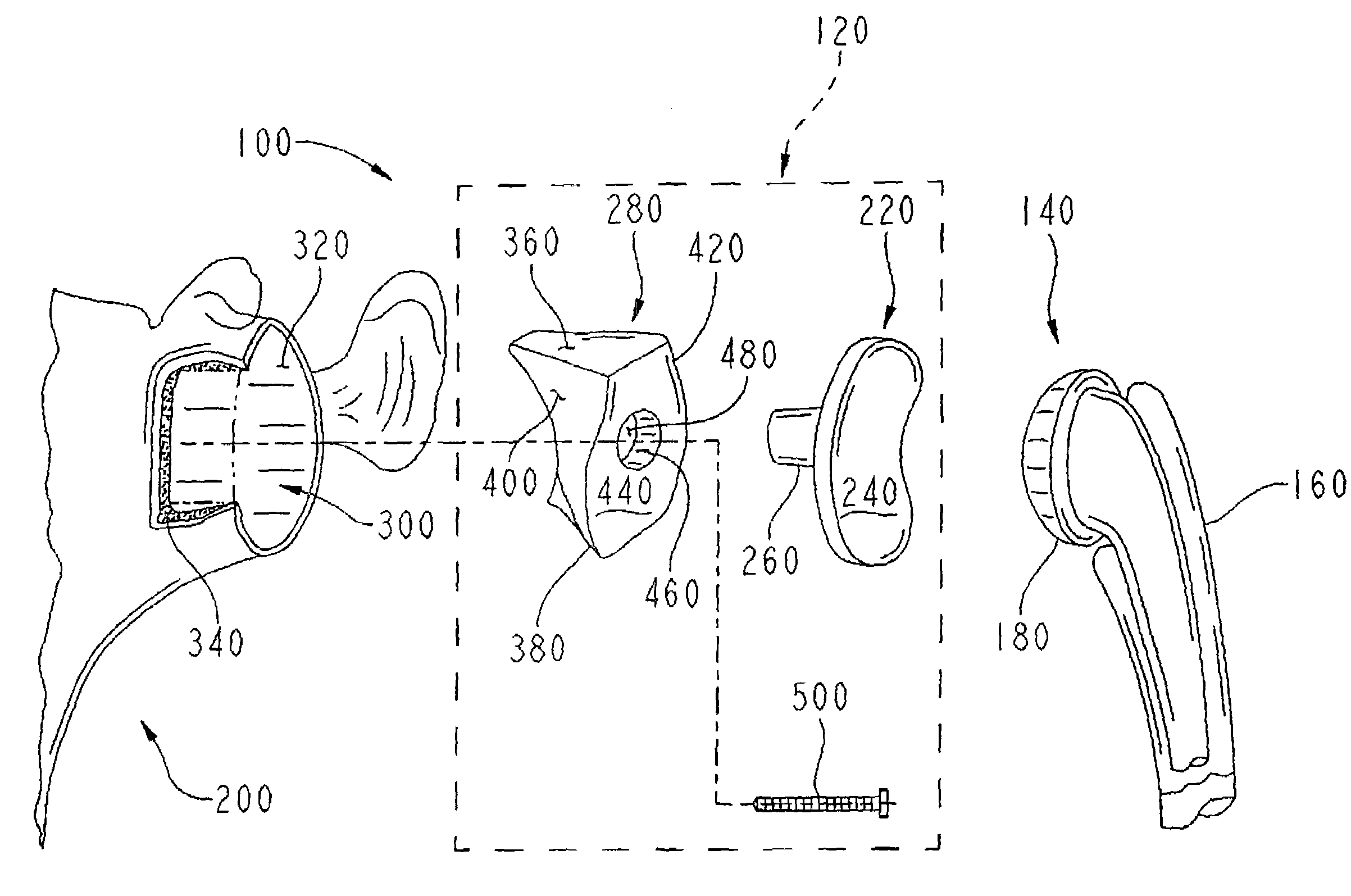
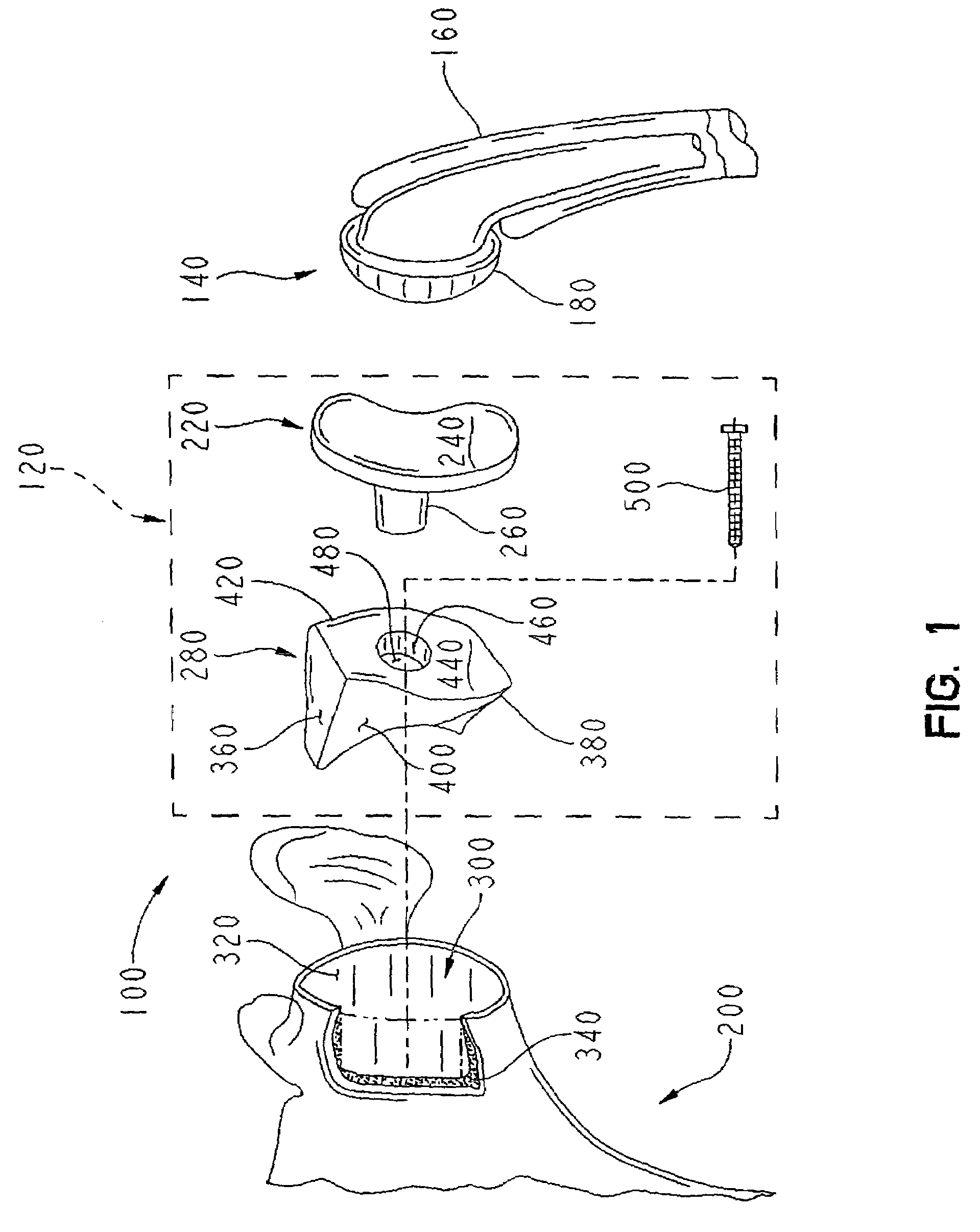
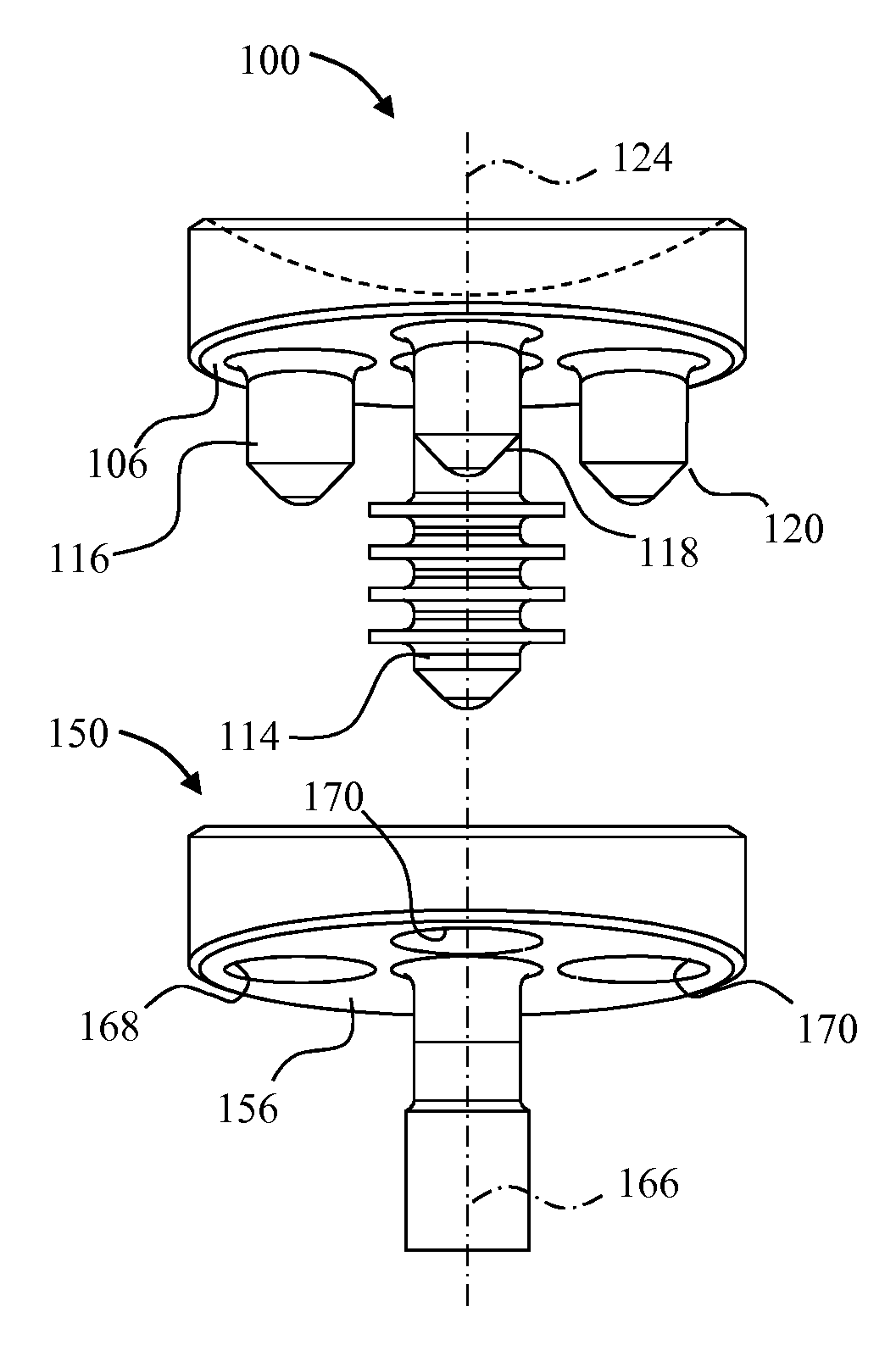
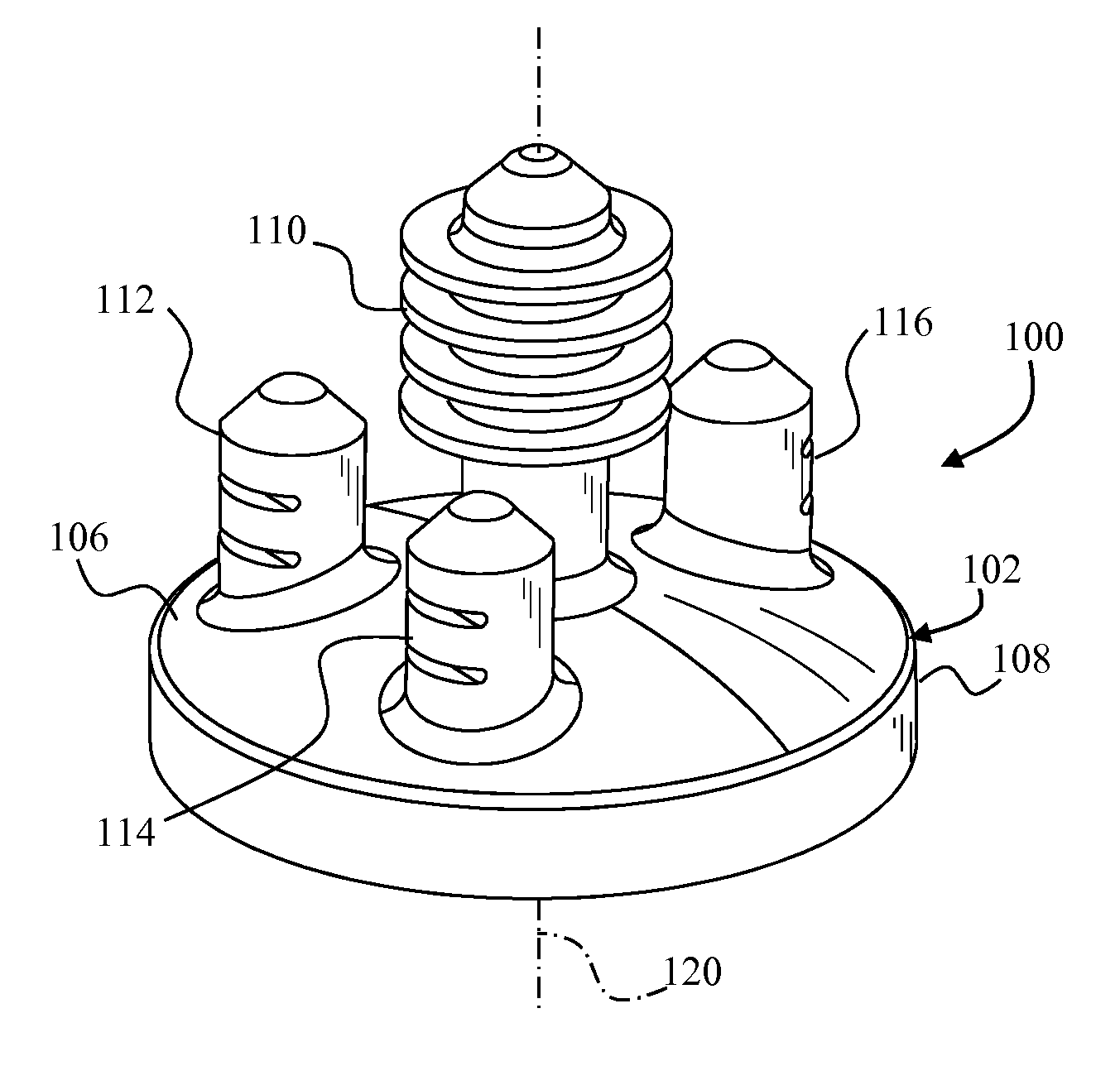
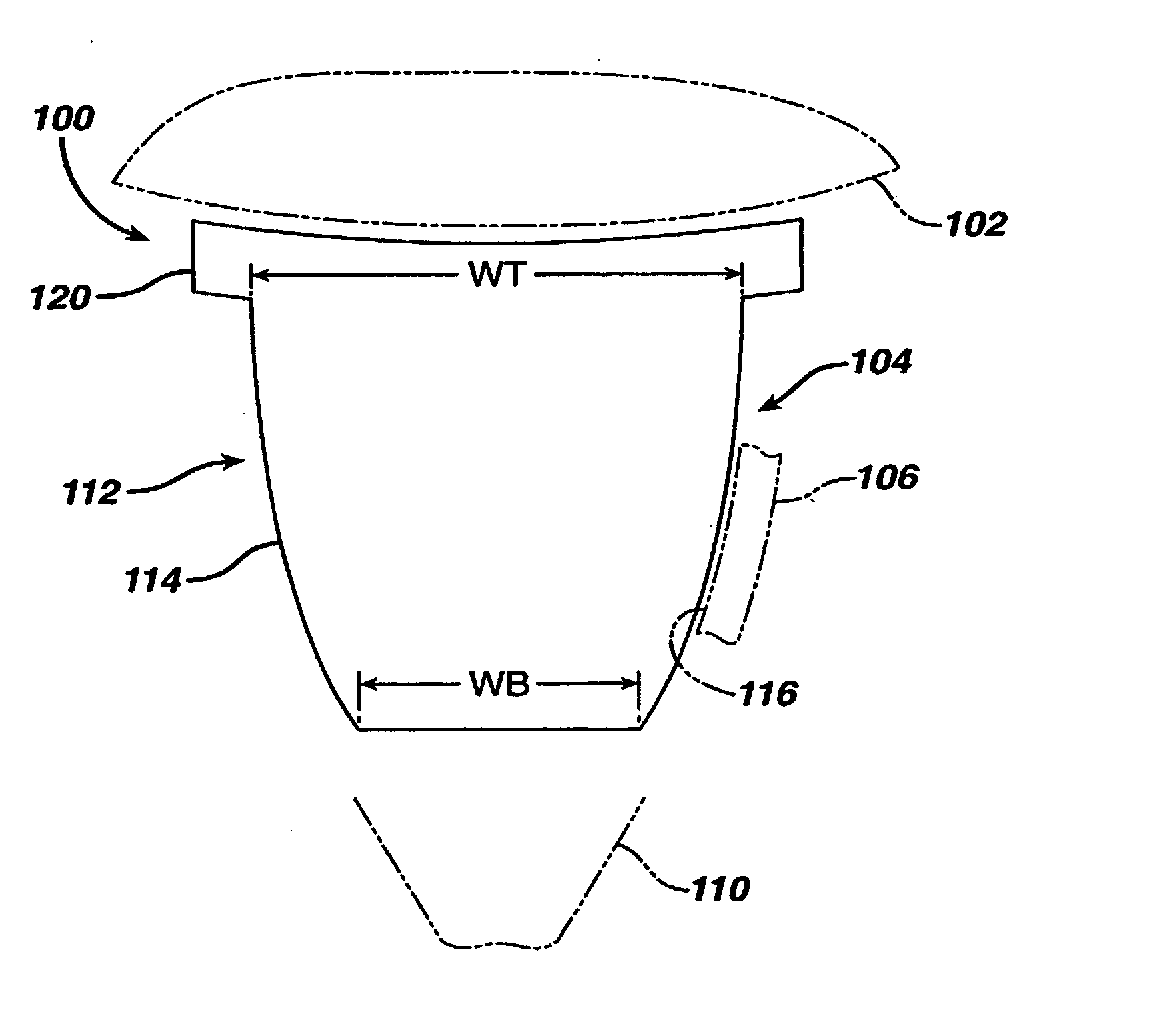
A glenoid component (100) for use with a prosthetic humeral component (102) for use in performing shoulder arthroplasty is provided. The glenoid component (100) may be fitted at least partially into a cavity (104) formed in the glenoid vault (106). The glenoid component (112) includes a body (112) having a stem portion (114) for inserting at least partially into the cavity (104) formed in the glenoid vault (106). The stem portion (114) cooperates with the interior wall (116) of the cavity (104) formed in the glenoid vault (106). The body (112) has a bearing portion (120) for articulating cooperation with the prosthetic humeral component (102).
Owner:DEPUY SYNTHES PROD INC
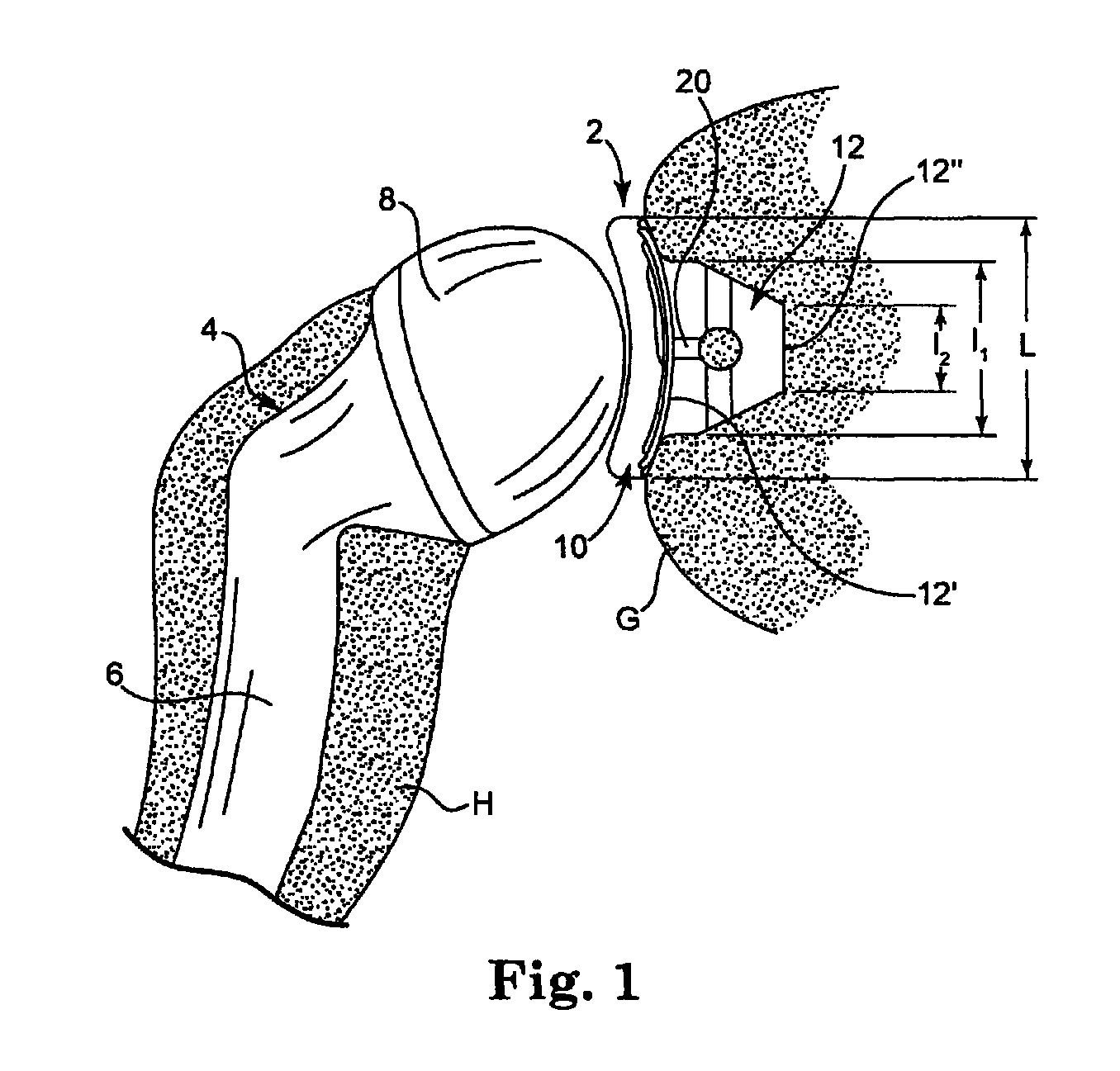
Dual-radius glenoid prosthetic component for total shoulder arthroplasty
InactiveUS6875234B2Reduce loadPowerfulJoint implantsShoulder jointsArticular surfacesTotal hip arthroplasty
A dual-radius glenoid component of a shoulder joint prosthesis for use in total shoulder arthroplasty has two radii of curvature. At substantially the center of the articulating surface a first non-conforming radius of curvature is provided, while a second conforming radius of curvature, smaller than that of the first radius of curvature and more closely conforming to the curvature of the humeral component, is disposed about the periphery of the articulating surface. The central non-conforming radius of curvature provides reduced constraint of the humeral component. In contrast, the conforming peripheral radius of curvature provides maximum constraint of the humeral component. When the humeral component is substantially centered on the articulating surface reduced constraint is placed on the prosthesis and less load is transferred to the bone-implant interface. As the humeral component translates towards the periphery, constraint on the prosthesis increases to prevent dislocation.
Owner:NEW YORK SOC FOR THE RUPTURED & CRIPPLED MAINTAINING THE HOSPITAL FOR SPECIAL SURGERY
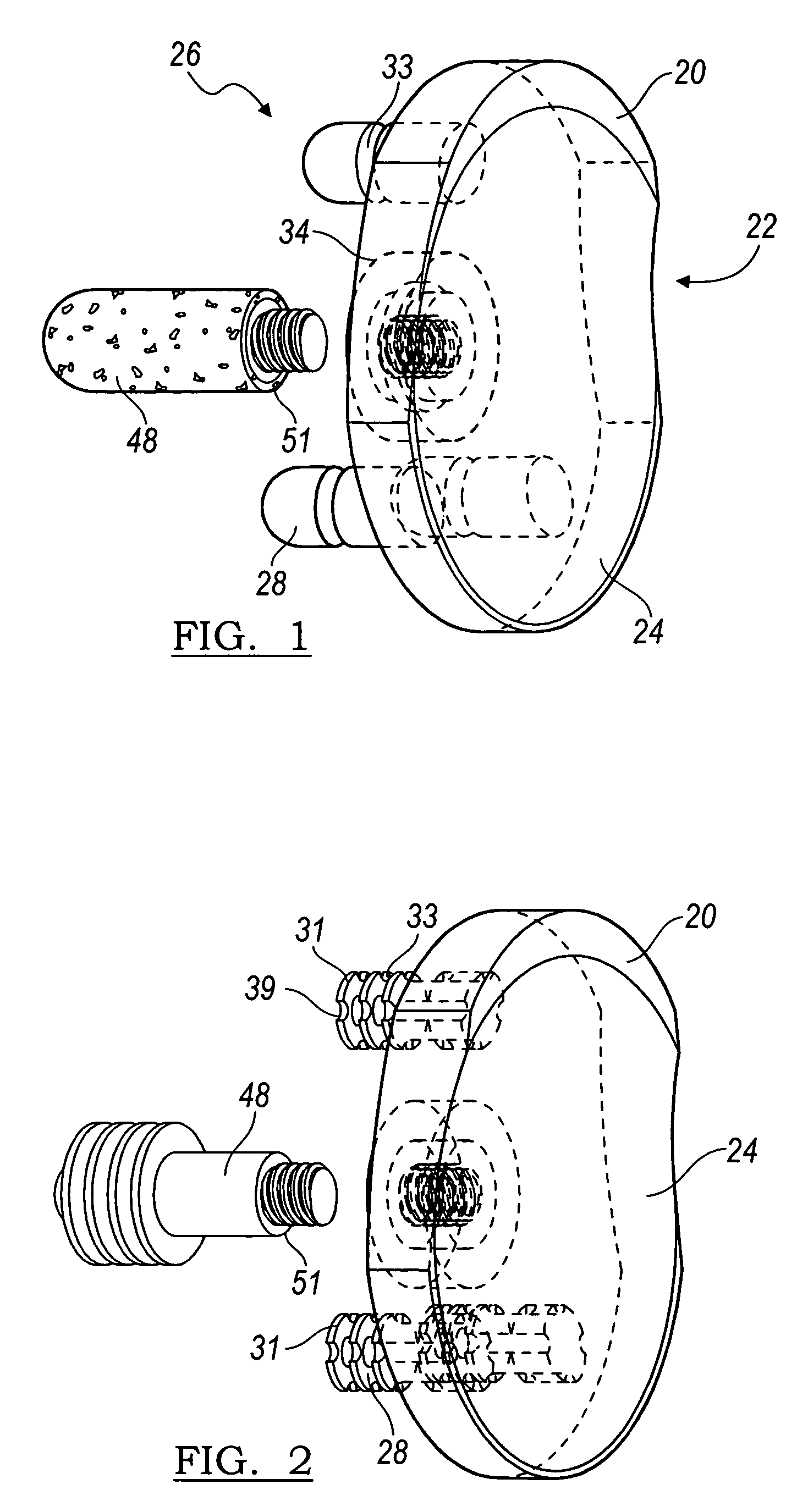
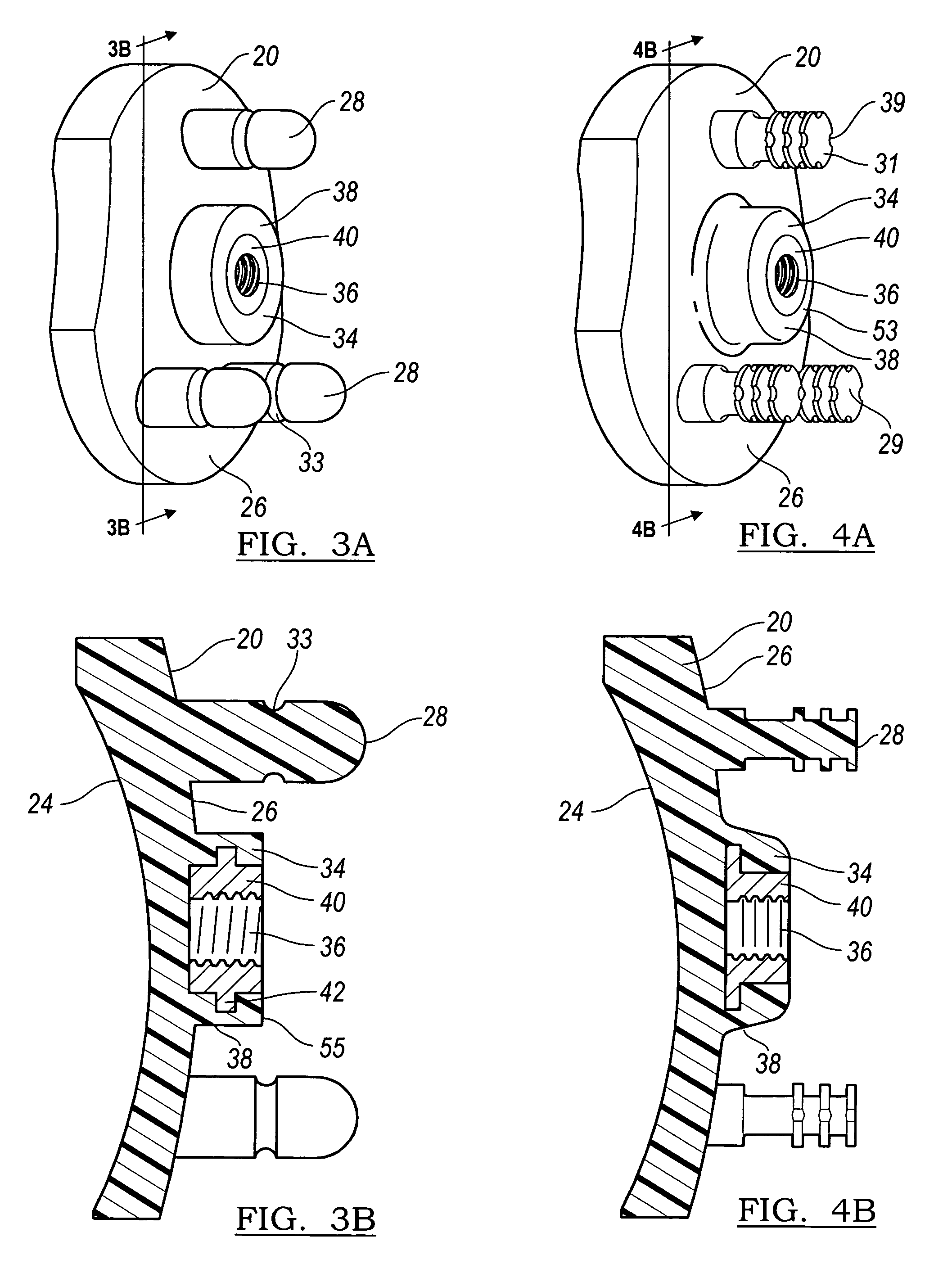
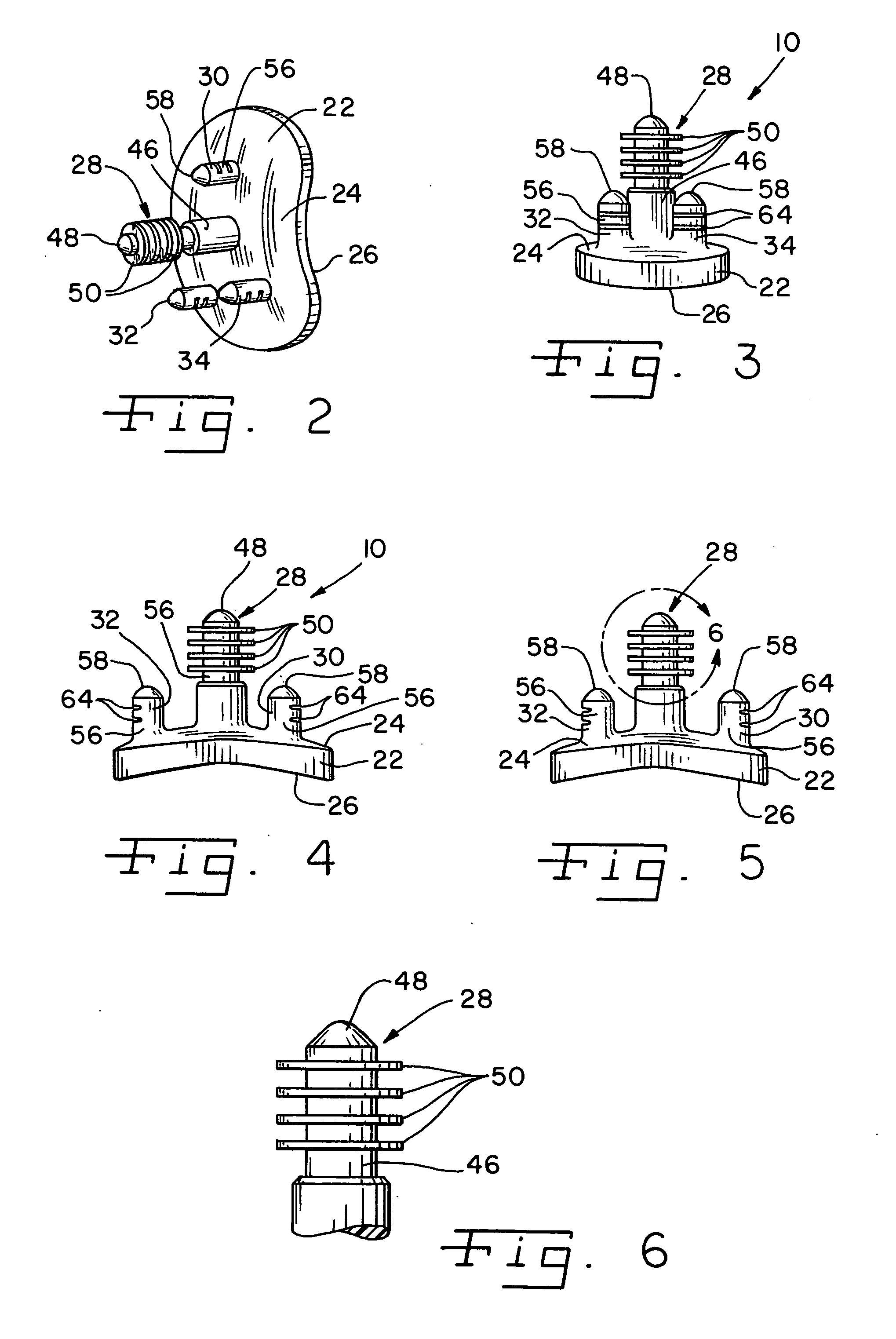
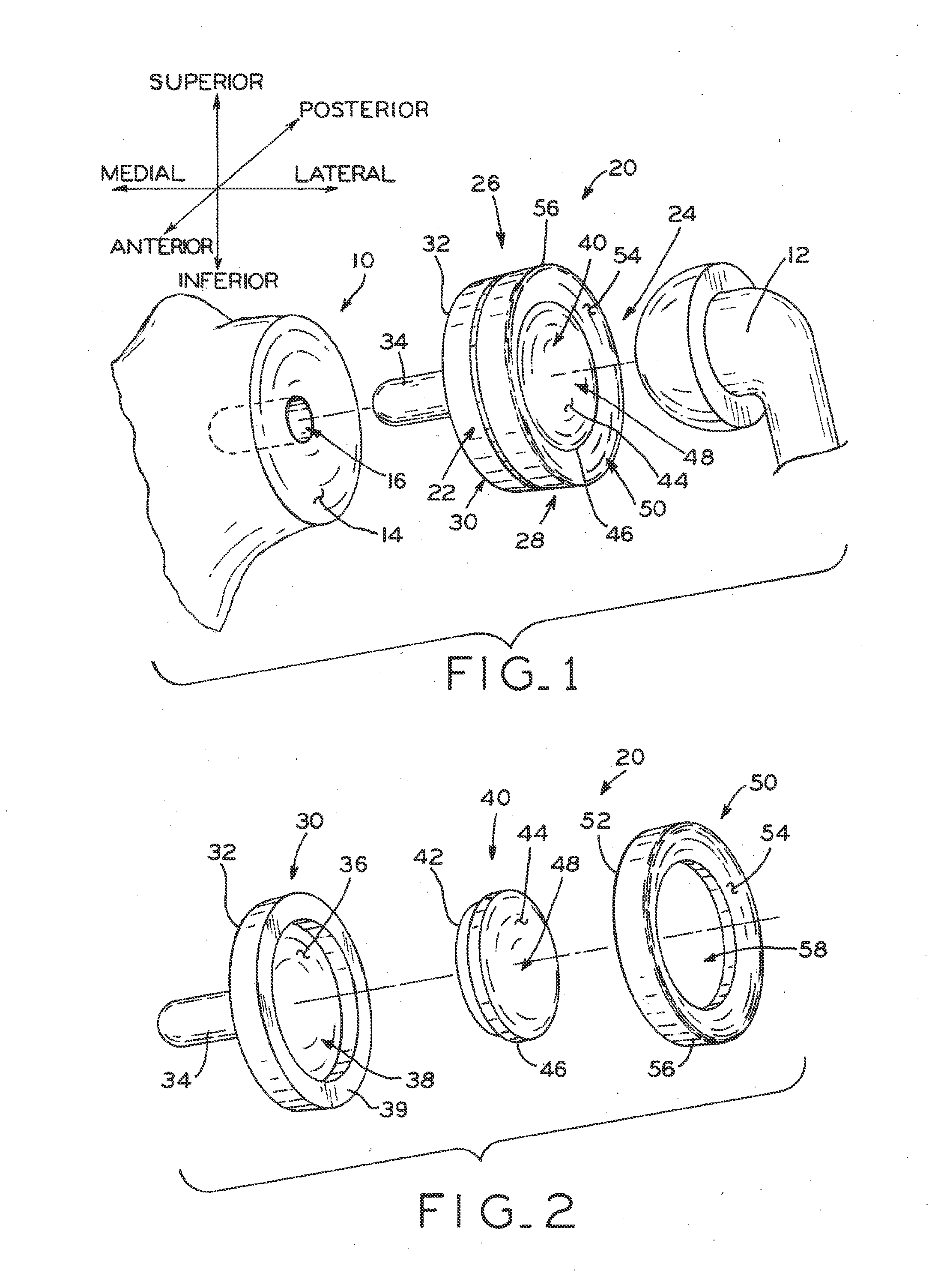
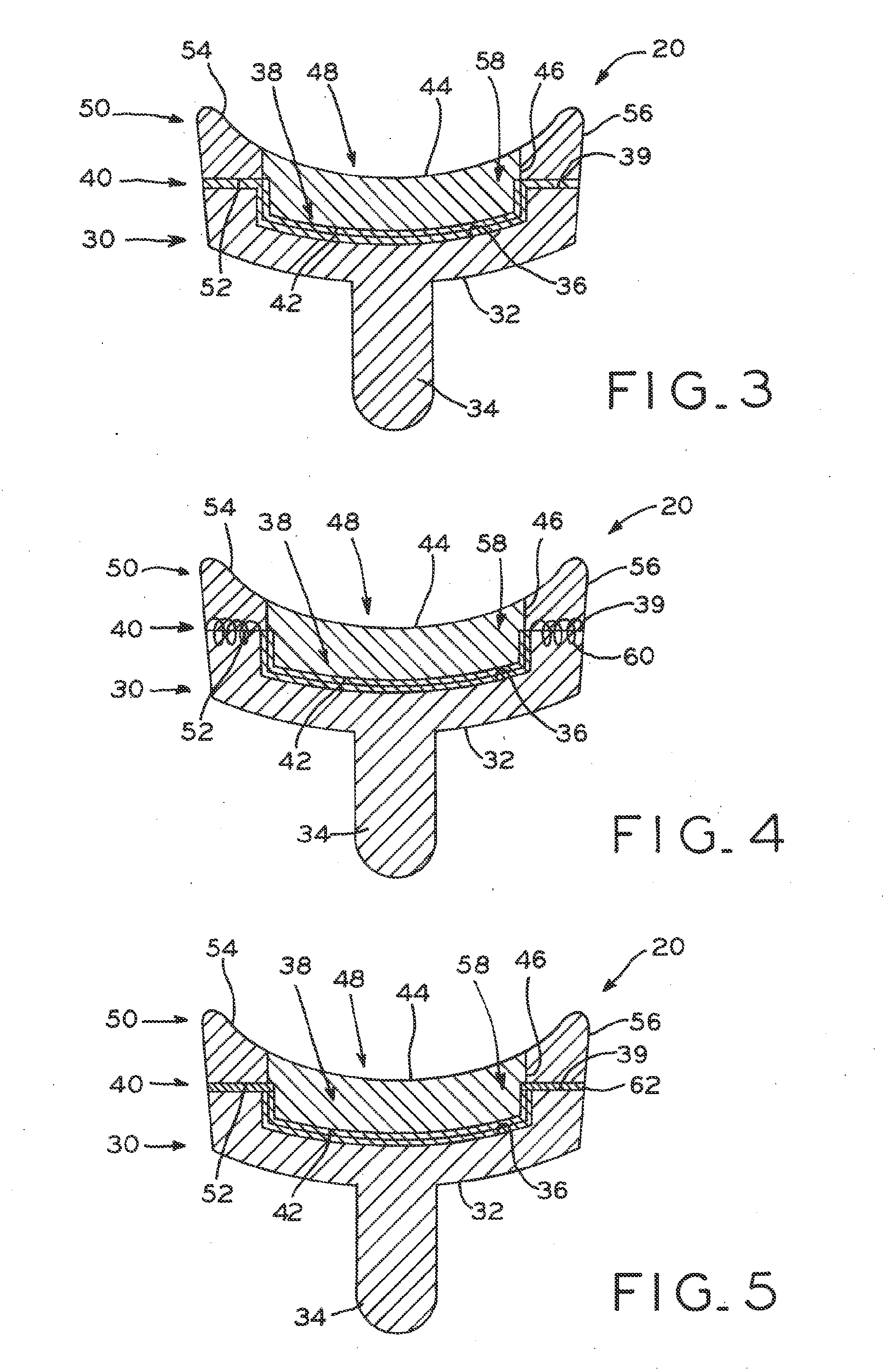
Modular center pegged glenoid
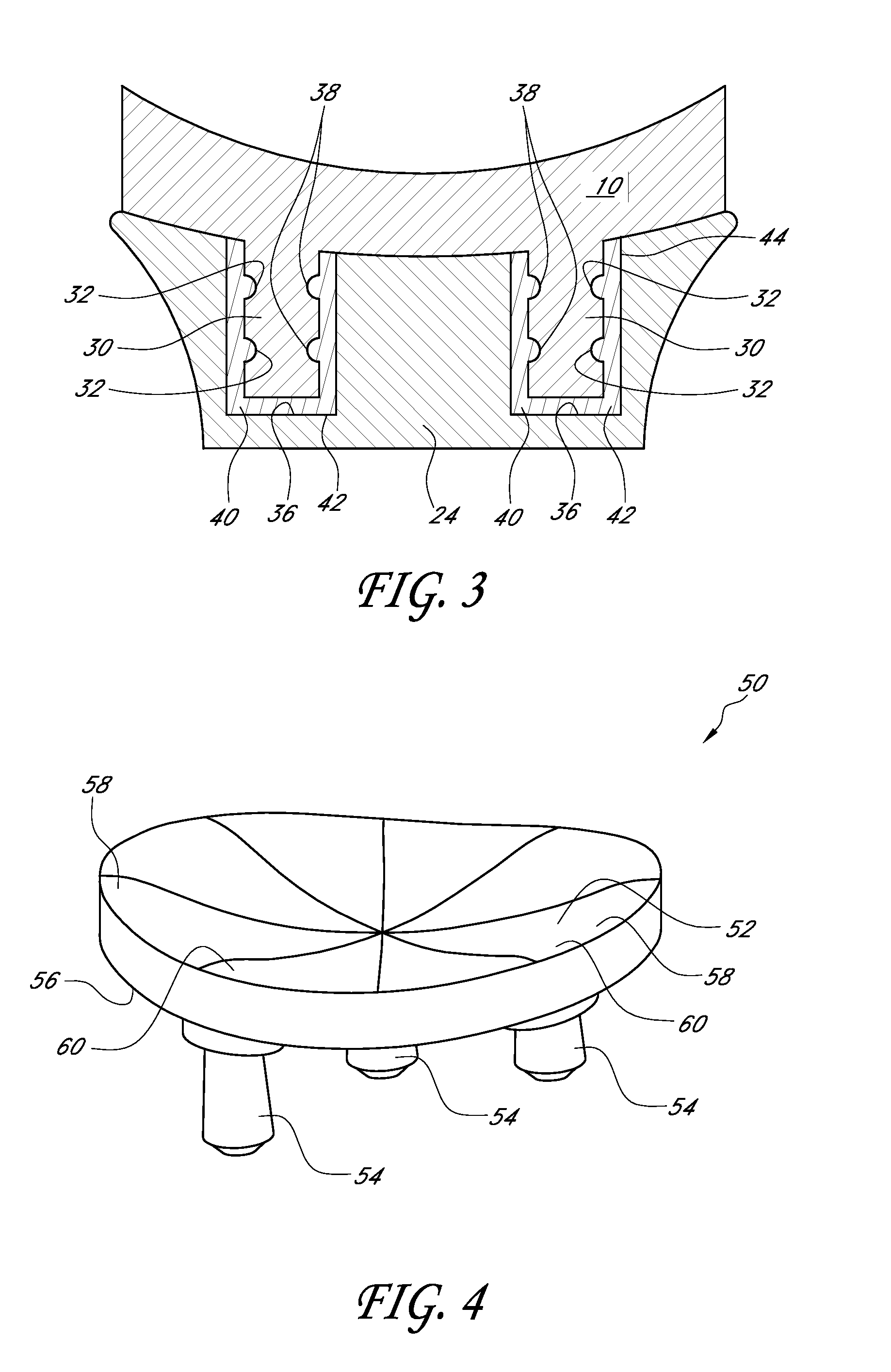
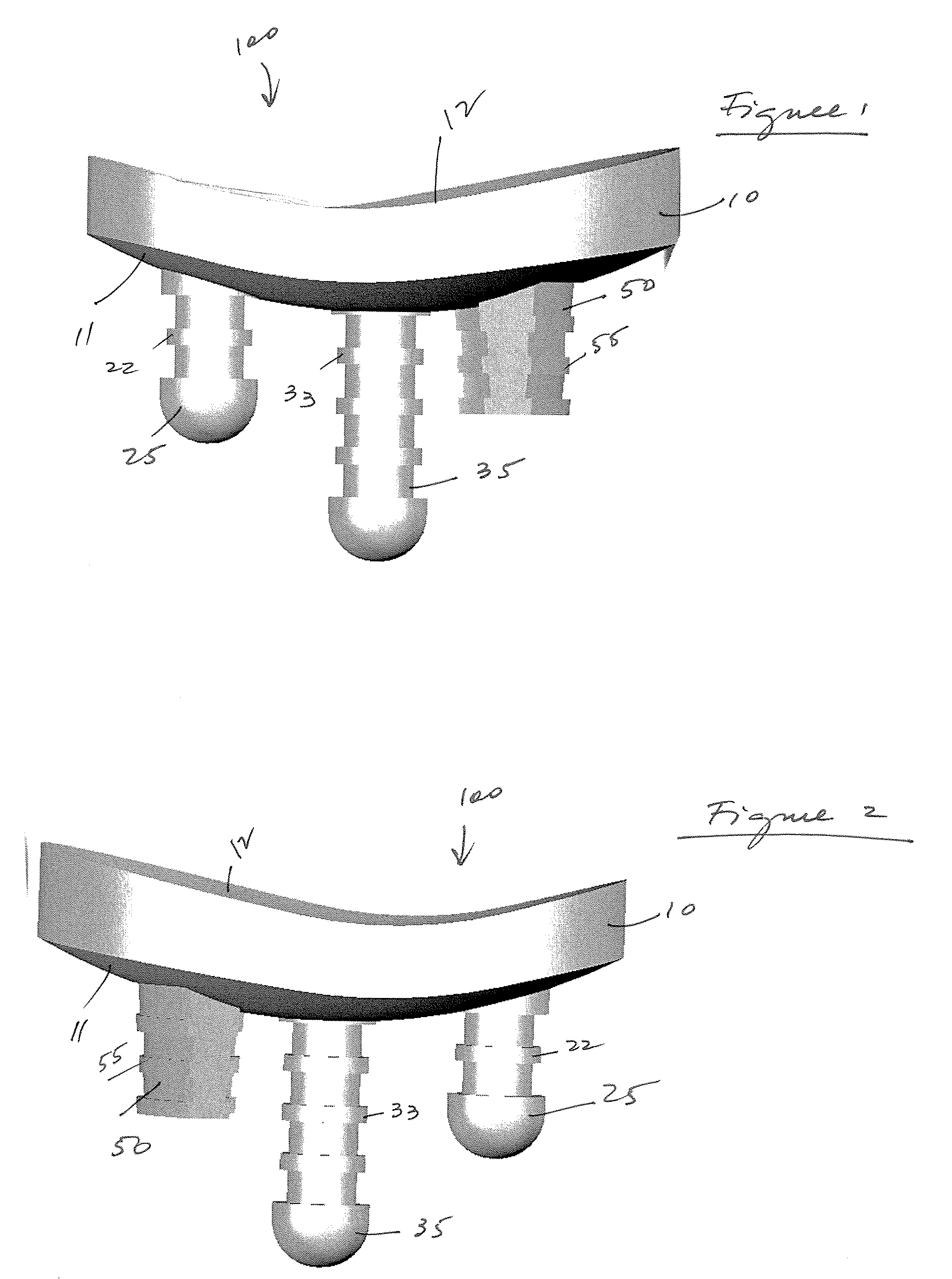
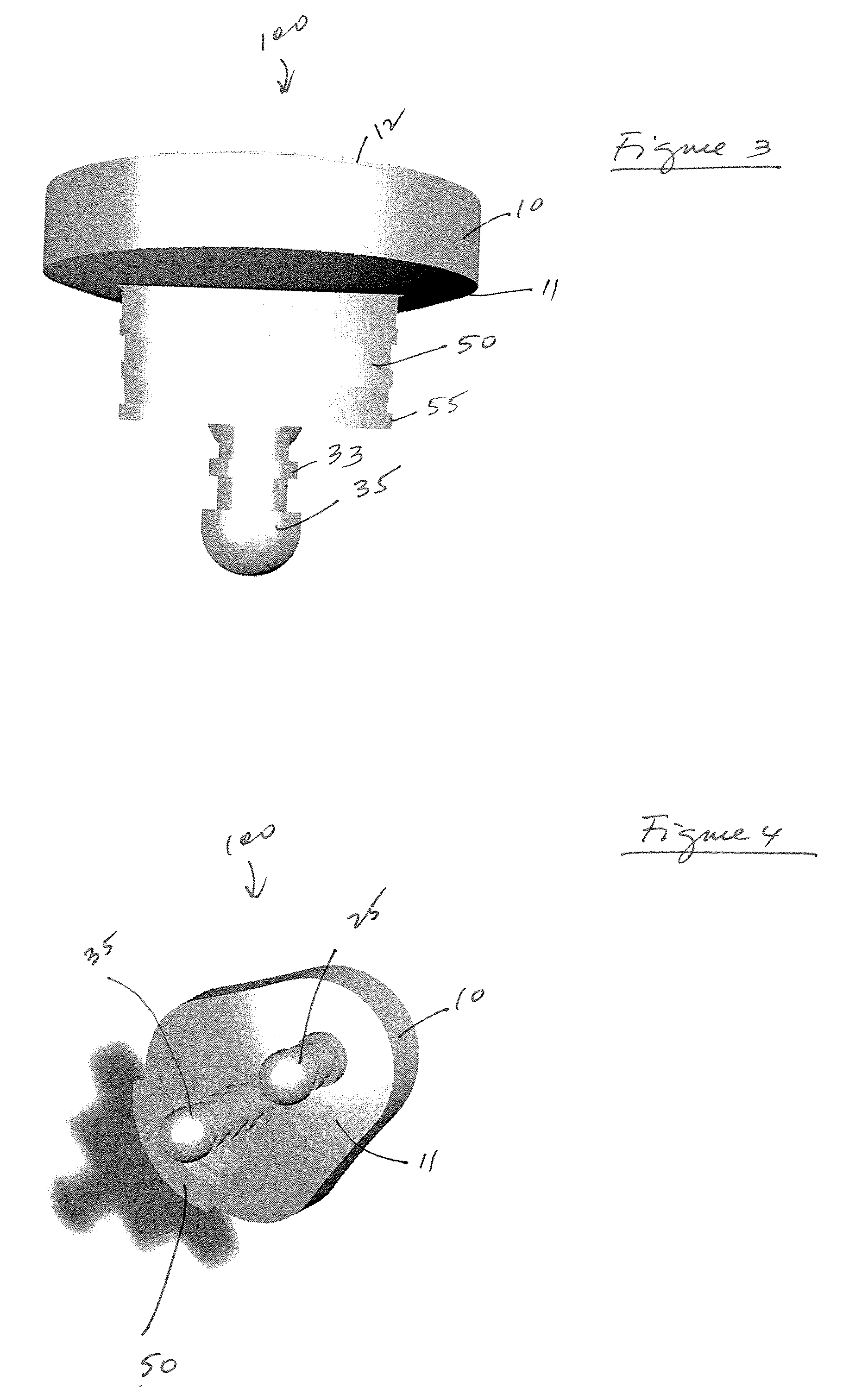
A glenoid component used for shoulder arthroplasty is adapted to be implanted into a scapula and engaged by a head of a humeral component. The glenoid component includes a body having a first articulating surface and a second medial surface opposite to the first articulating surface. The first articulating surface is adapted to engage with a humeral head. A plurality of fixed pegs each have a first end adapted to engage a cavity formed in the scapula and a second end extending from the medial surface. A central peg fixation mechanism is provided that is configured to couple an optional central fixation peg to the medial surface.
Owner:BIOMET MFG CORP
Reverse shoulder prosthesis
Various embodiments of the present invention relate to an apparatus and method for reverse shoulder arthroplasty (e.g., reverse total shoulder arthroplasty). In one specific example, a glenoid component used to resurface the scapula may be provided. Of note, unlike traditional total shoulder arthroplasty the glenoid component in a reverse shoulder is convex rather than concave; it acts as a physical stop to prevent the superior migration of the humeral head—a typical occurrence in patients suffering from rotator cuff tear arthropathy (CTA).
Owner:EXACTECH INC
Modular center pegged glenoid
A glenoid component used for shoulder arthroplasty is adapted to be implanted into a scapula and engaged by a head of a humeral component. The glenoid component includes a body having a first articulating surface and a second medial surface opposite to the first articulating surface. The first articulating surface is adapted to engage with a humeral head. A plurality of fixed pegs each have a first end adapted to engage a cavity formed in the scapula and a second end extending from the medial surface. A central peg fixation mechanism is provided that is configured to couple an optional central fixation peg to the medial surface.
Owner:BIOMET MFG CORP
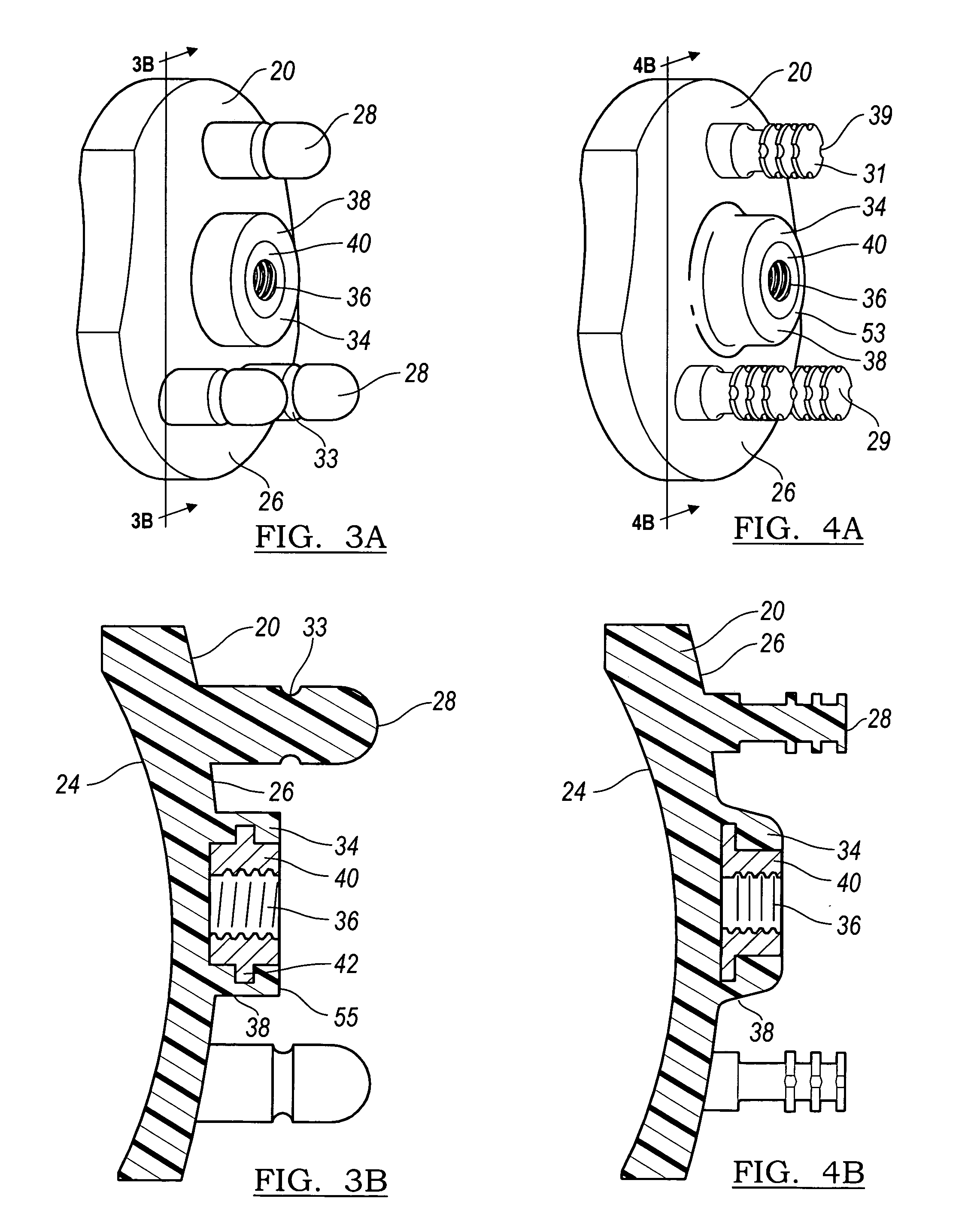
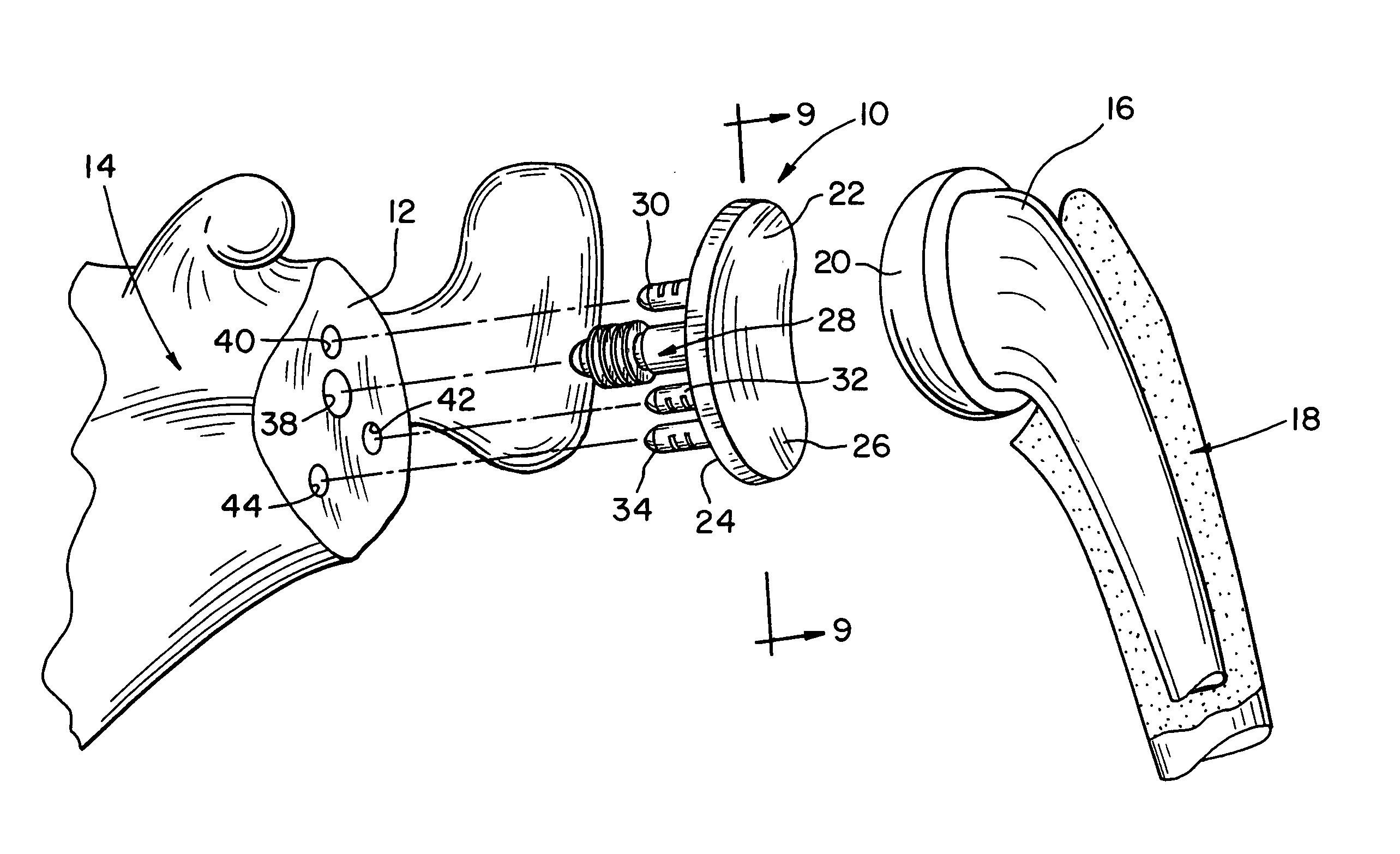
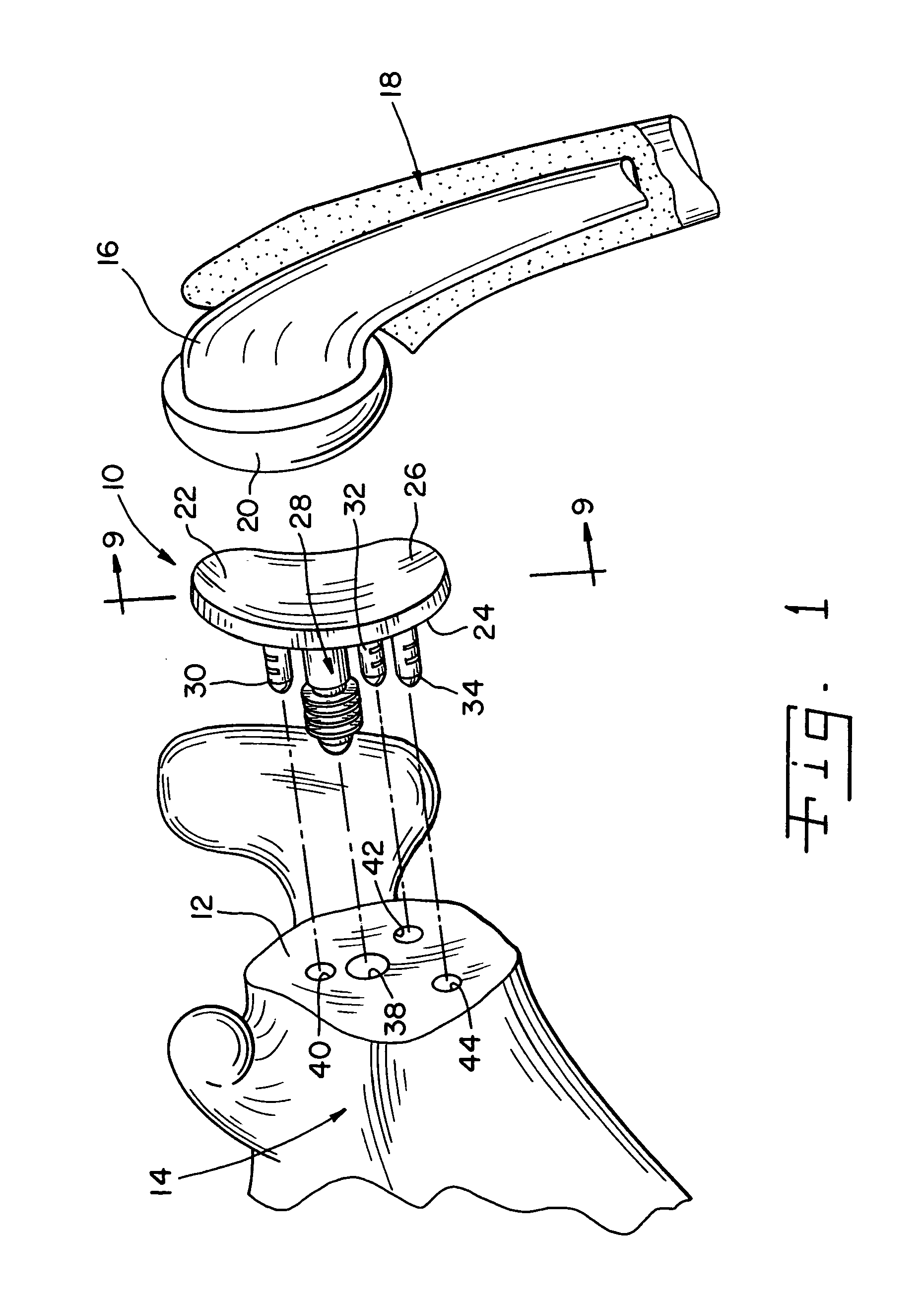
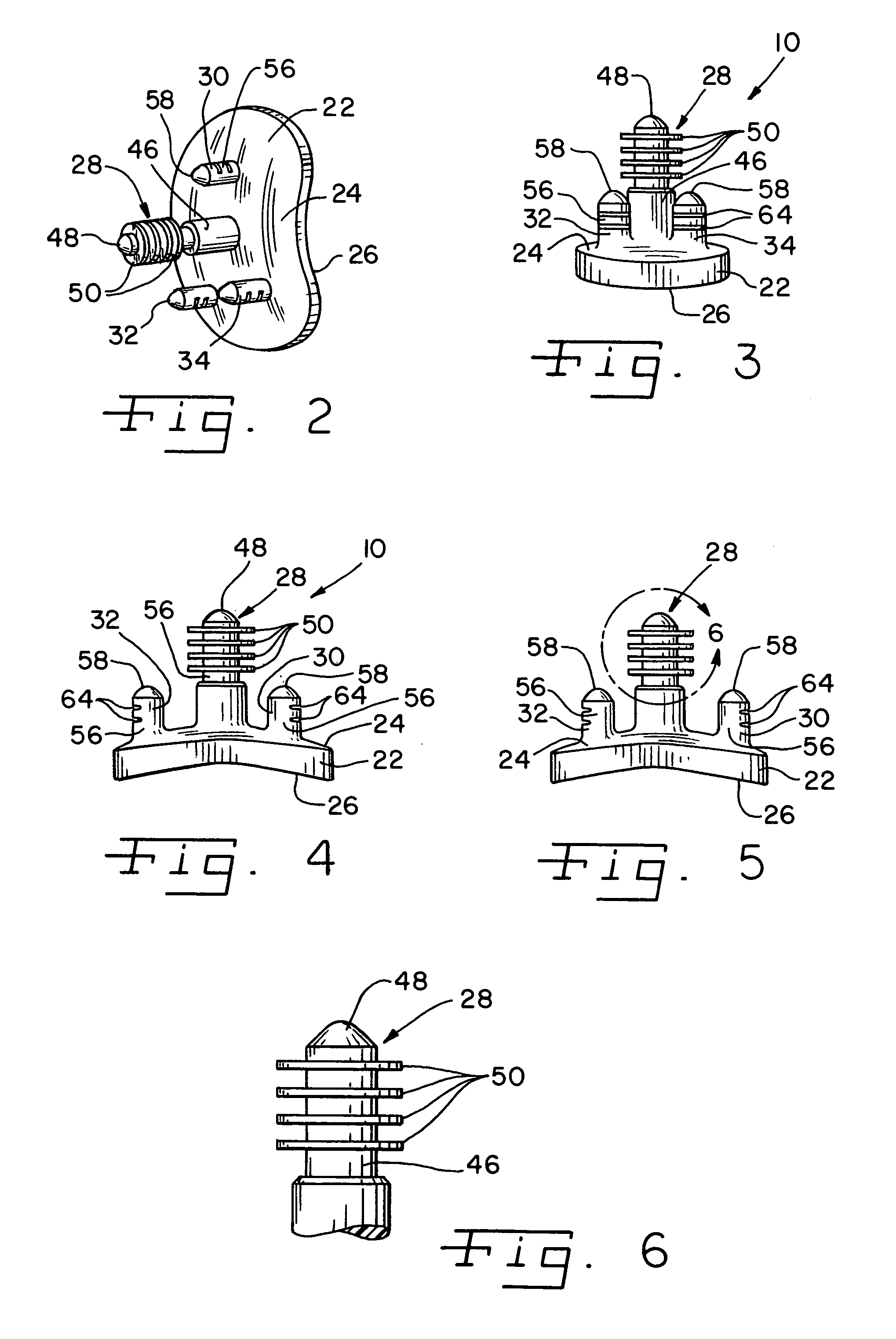
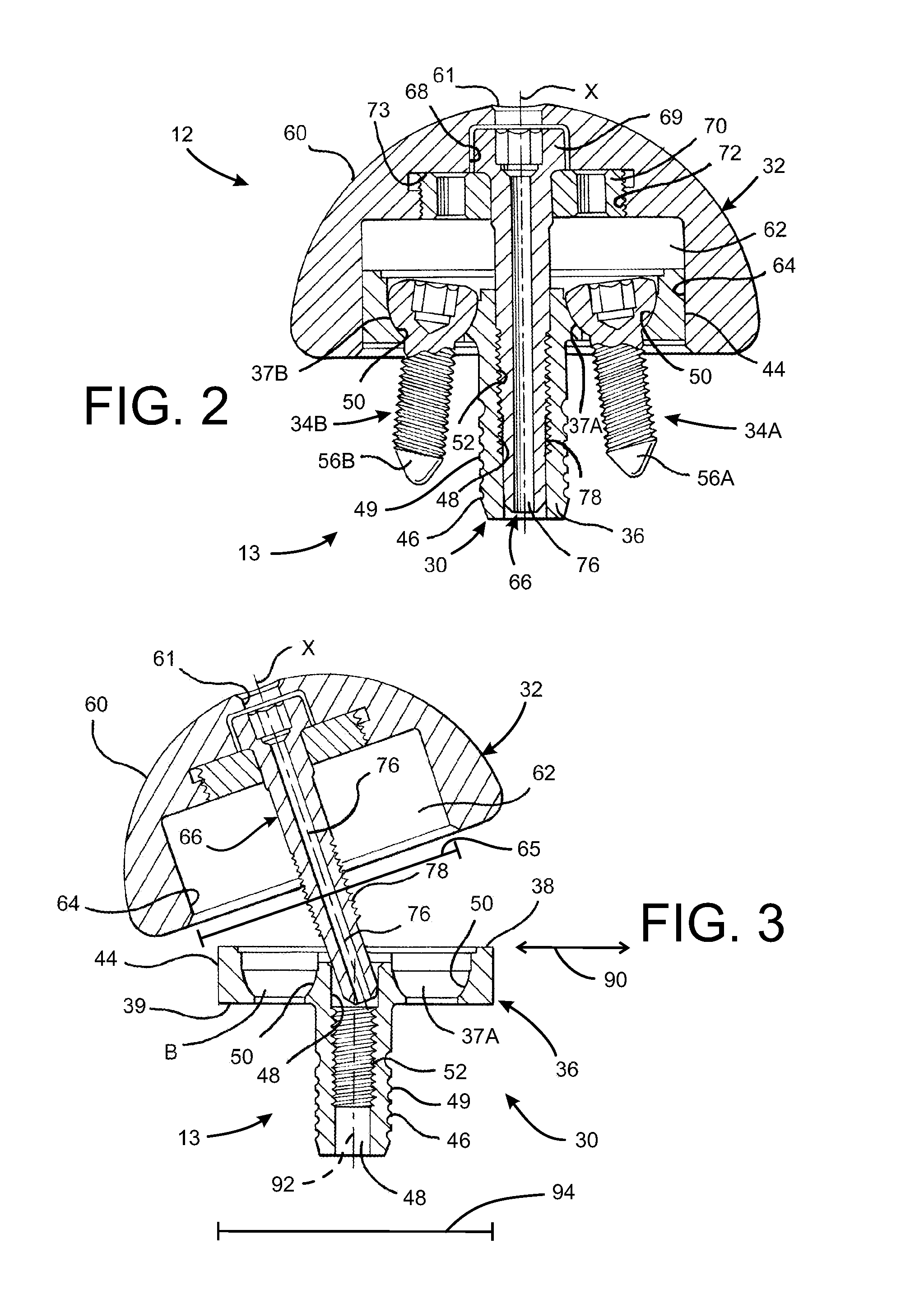
Prosthetic glenoid component
A prosthetic glenoid component for attachment to a scapula to provide a bearing for a humeral head in a shoulder prosthesis has a one-piece bearing element having a concave lateral bearing surface for contact with the humeral head with which it is to be used. An opposing relatively hard medial surface of the bearing element is provided for attachment to a scapula. The lateral surface is a soft low modulus concave lateral bearing surface extends around the periphery of the bearing element and increases its thickness to provide a deformable rim to simulate the labrum in an anatomical glenoid. The bearing element preferably has two affixation pegs which project from the medial face thereof, one at a superior position which projects in a superior direction and the other which is located in an inferior position and which projects in an inferior direction which is angled in relation to the medial-lateral direction.
Owner:STRYKER EURO OPERATIONS HLDG LLC
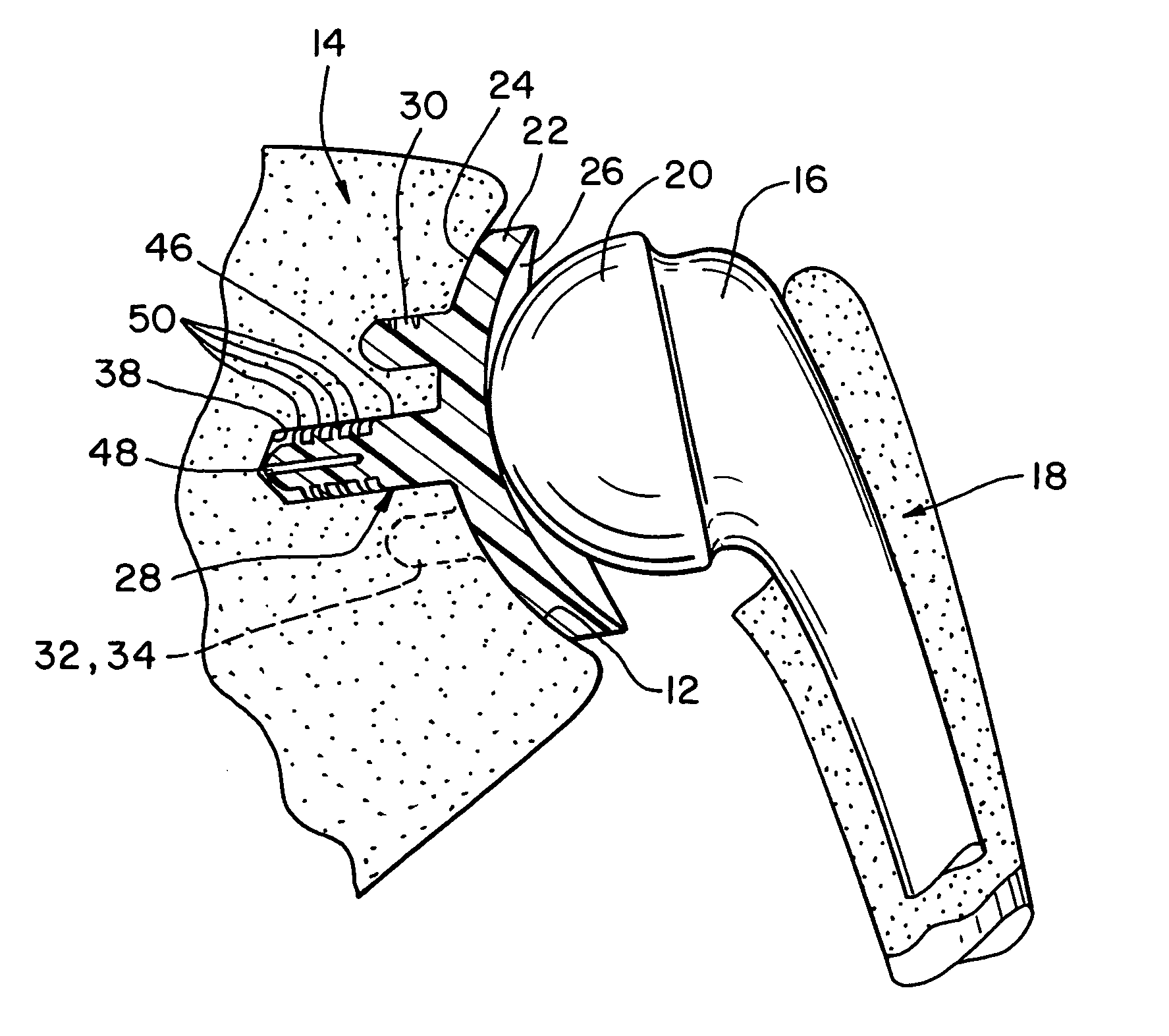
Semiconstrained shoulder prosthetic for treatment of rotator cuff arthropathy
InactiveUS20060079963A1Inhibition of translationProvide stabilityJoint implantsNon-surgical orthopedic devicesKeelAcromion
A glenoid component for treatment of rotator cuff arthropathy includes attachment to the coracoid process by a pin or post imbedded into a hole formed in the coracoid process. The glenoid component preferably also has a keel for extending into the glenoid fossa and protrusions such as ridges cemented to the acromion process. This way, an attachment point is preferably provided in / on the coracoid process, the acromion process, and the glenoid fossa, and at least two of the attachment points include a protrusion extending into a hole / slot drilled or otherwise formed in the bone. A jig is used for guiding / drilling into the bone, wherein the jig has both a glenoid fossa drill guide and a coracoid drill guide. The coracoid drill guide includes structure that abuts against opposing sides of the base of the coracoid process to prevent rotation of the jig relative to the scapula.
Owner:HANSEN REGAN
Reverse shoulder prosthesis
Various embodiments of the present invention relate to an apparatus and method for reverse shoulder arthroplasty (e.g., reverse total shoulder arthroplasty). In one specific example, a glenoid component used to resurface the scapula may be provided. Of note, unlike traditional total shoulder arthroplasty the glenoid component in a reverse shoulder is convex rather than concave; it acts as a physical stop to prevent the superior migration of the humeral head—a typical occurrence in patients suffering from rotator cuff tear arthropathy (CTA).
Owner:EXACTECH INC
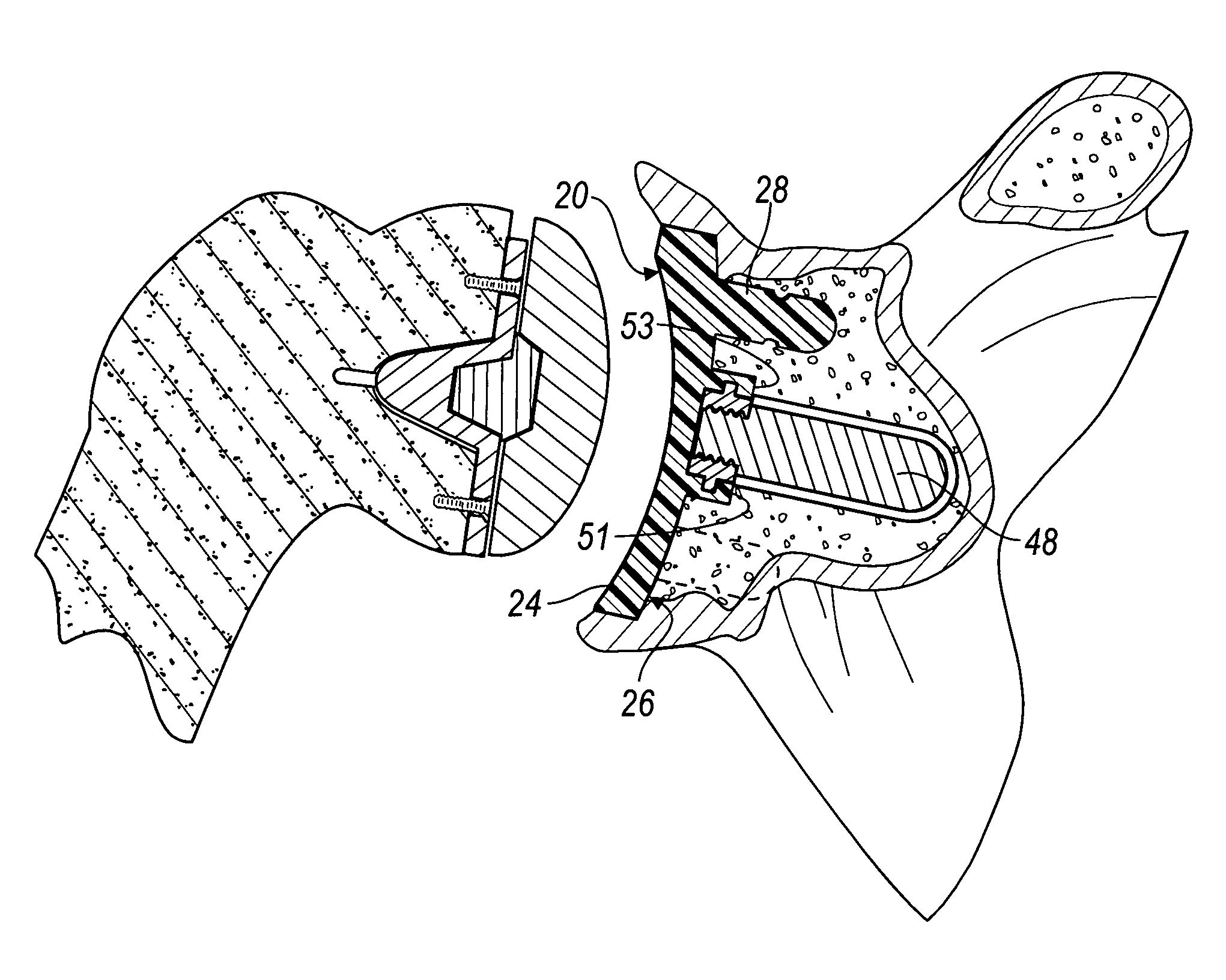
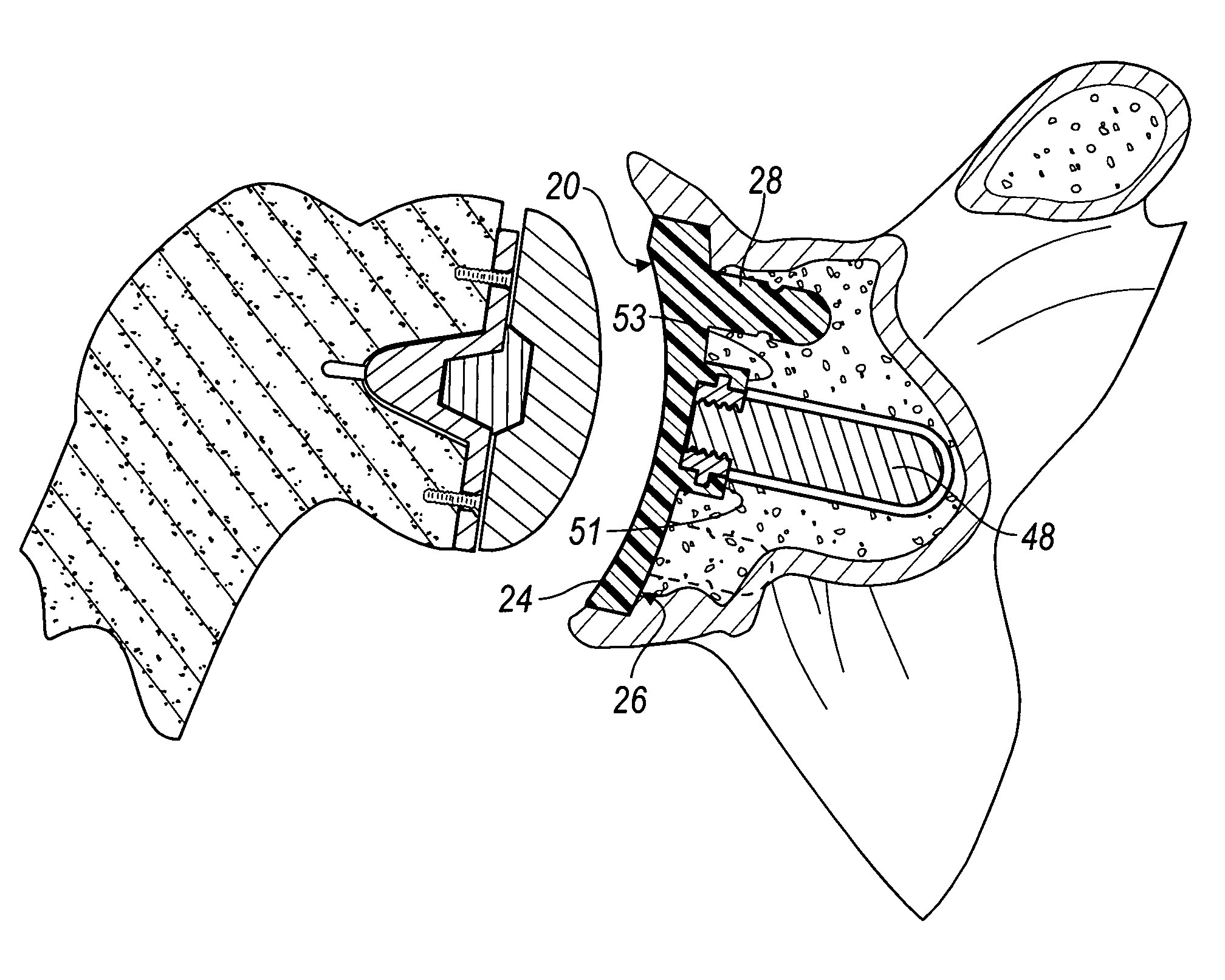
Method for securing a glenoid component to a scapula
A medical procedure includes providing a prosthesis which includes (i) a body portion having a rear surface and a bearing surface, and (ii) an anchor peg including a shaft extending from the rear surface of the body portion, wherein the anchor peg includes a plurality of outwardly extending fins secured to the shaft, and further wherein the plurality of outwardly extending fins each possesses a first diameter. The medical procedure further includes creating an anchor hole in a natural bone, the anchor hole possessing a second diameter which is less than the first diameter. In addition, the method procedure includes positioning the anchor peg within the anchor hole so that each of the plurality of outwardly extending fins deforms so as to possess (i) a concave side which faces an open end of the anchor hole, and (ii) a convex side that faces a closed end of the anchor hole.
Owner:DEPUY PROD INC
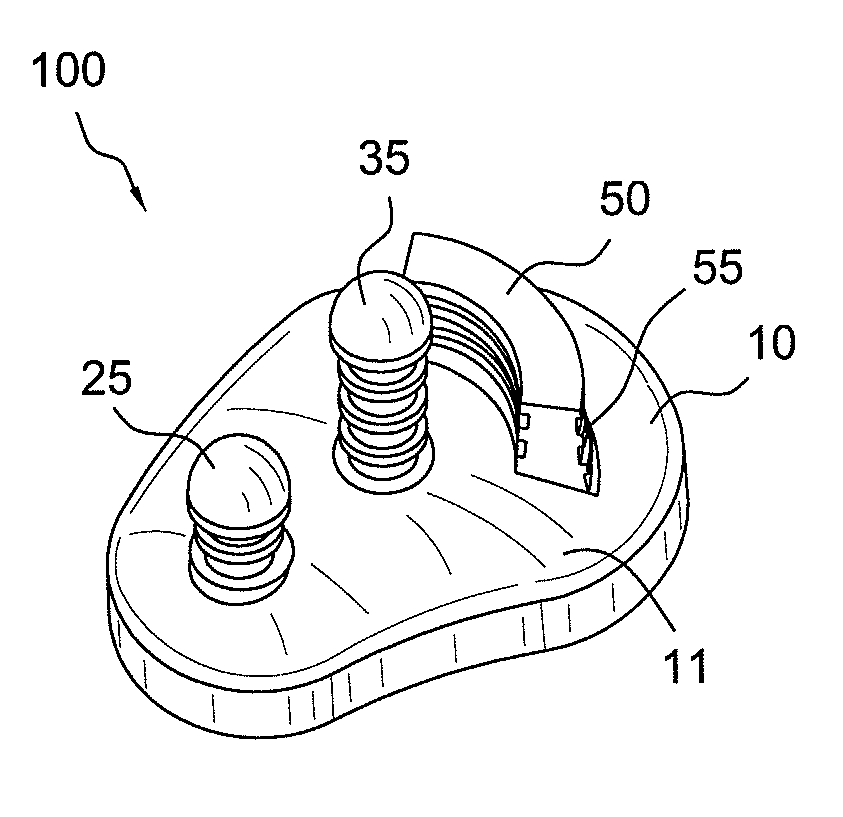
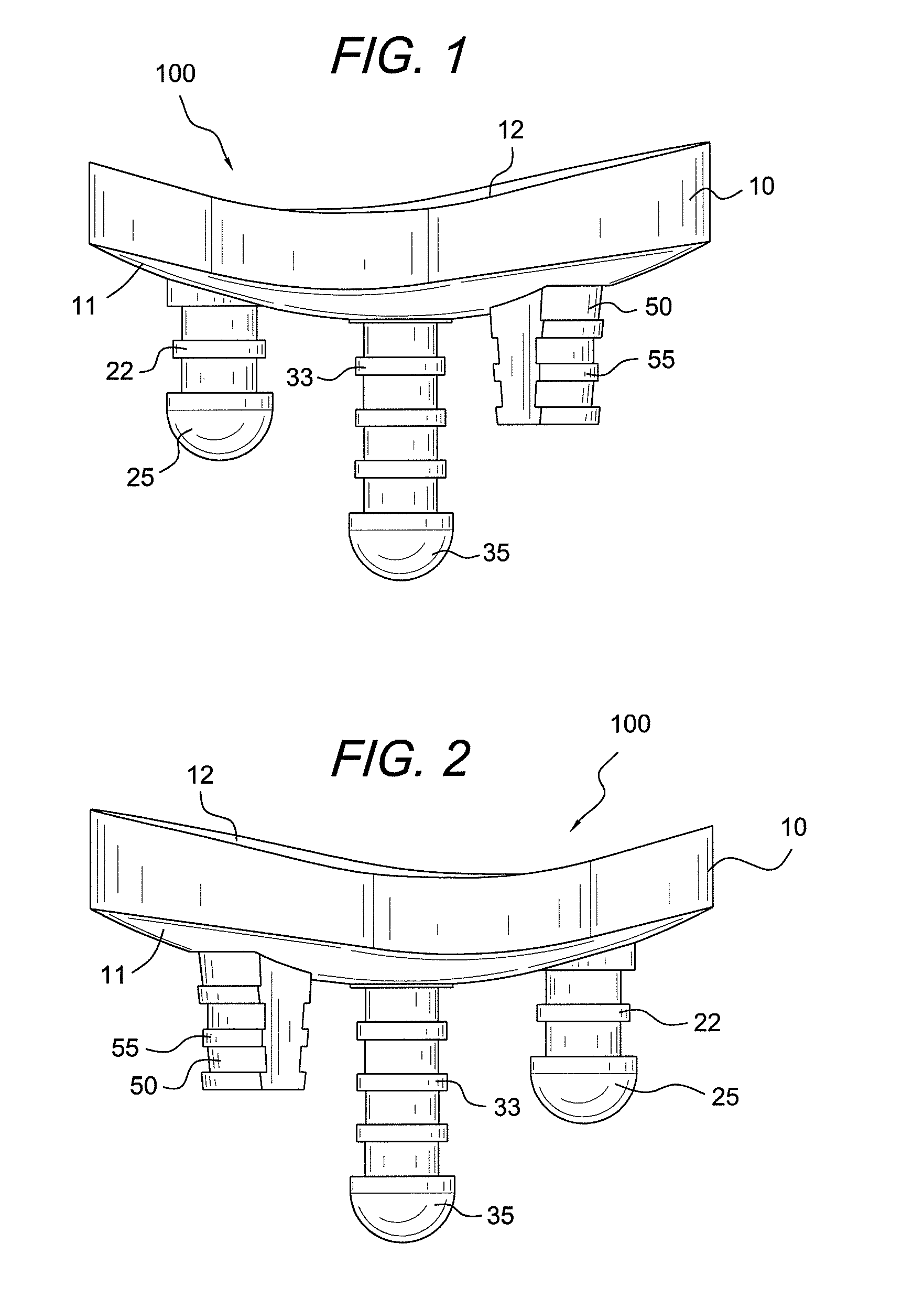
Glenoid component with improved fixation stability
ActiveUS20070225817A1Improve mobilityReduce stressJoint implantsShoulder jointsCement mantleArticular surfaces
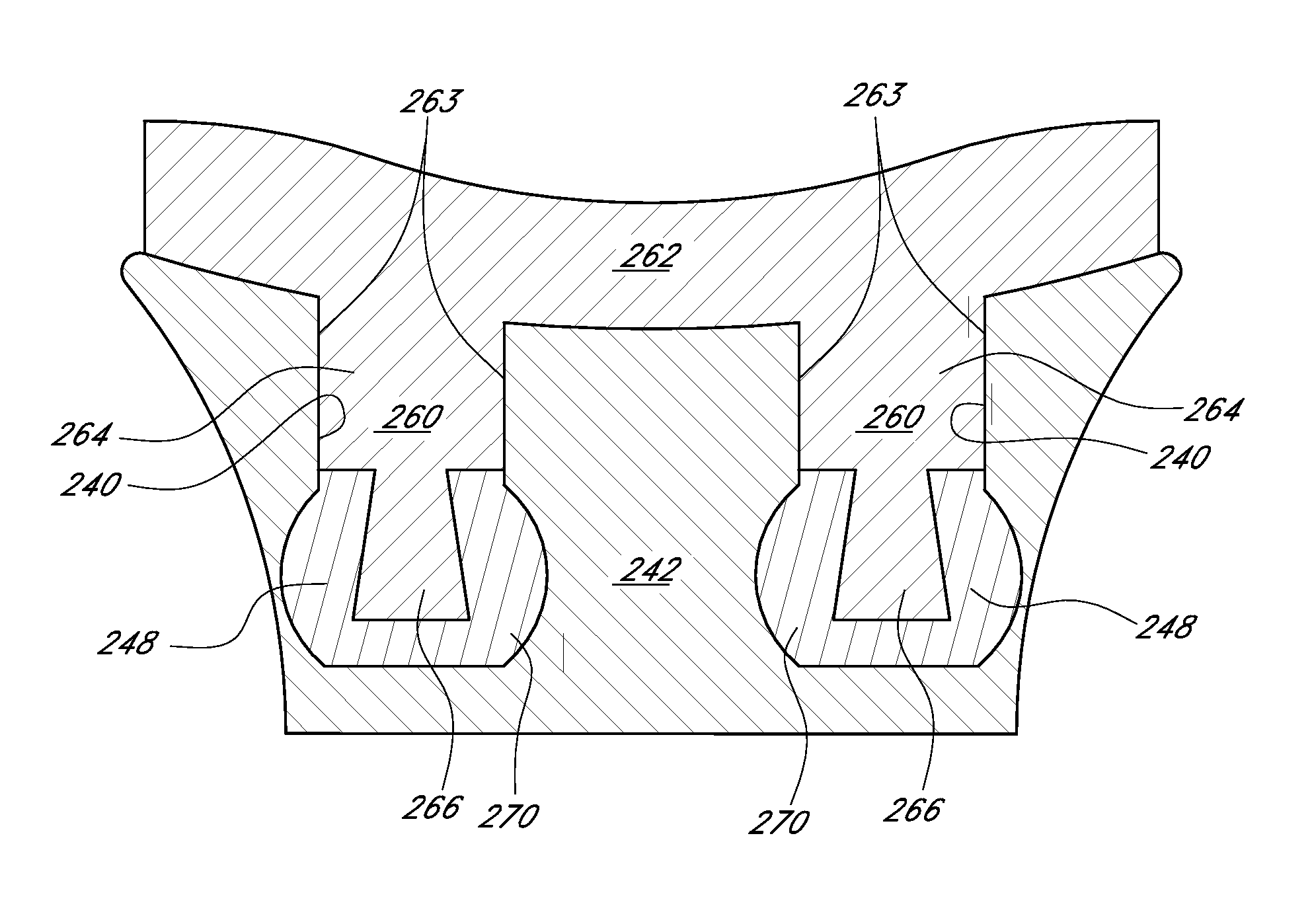
A glenoid component is provided to reduce glenoid loosening when implanted in orthopedic joint replacement / reconstruction, such for a shoulder. The glenoid component can include pegs or a keel and articulating surface geometry that uses complex, non-spherical geometry to recreate a level of constraint that is adequate, but not excessive, to thereby mitigate loosening of the glenoid component after implantation. In addition, some embodiments provide that peak stresses both within cement and at an interface of the cement and a supportive component can be reduced. Further, geometry of the pegs can allow stresses to be evenly applied to a cement mantle formed in the supportive component. Finally, the pegs can be configured to desired lengths in order to avoid placement in areas of the supportive component, for example, that have insufficient bone stock.
Owner:HOWMEDICA OSTEONICS CORP
Hybrid glenoid for shoulder arthroplasty
Glenoid prosthesis with a hybrid design (combining both peg and keel designs), and surgical methods for reconstitution of a shoulder joint. The hybrid design combines both peg and keel components into one device. The hybrid glenoid component of a shoulder prosthesis includes an oval body having a concave lateral articulating surface and an opposing convex medial surface. The medial surface is provided with a plurality of pegs and a single inferior protrusion. The pegs are used to attain both a superior location as well as a central location. The inferior protrusion provides stability in the superior-to-inferior translation of the humeral head, as well as rotational articulation of the same.
Owner:ARTHREX
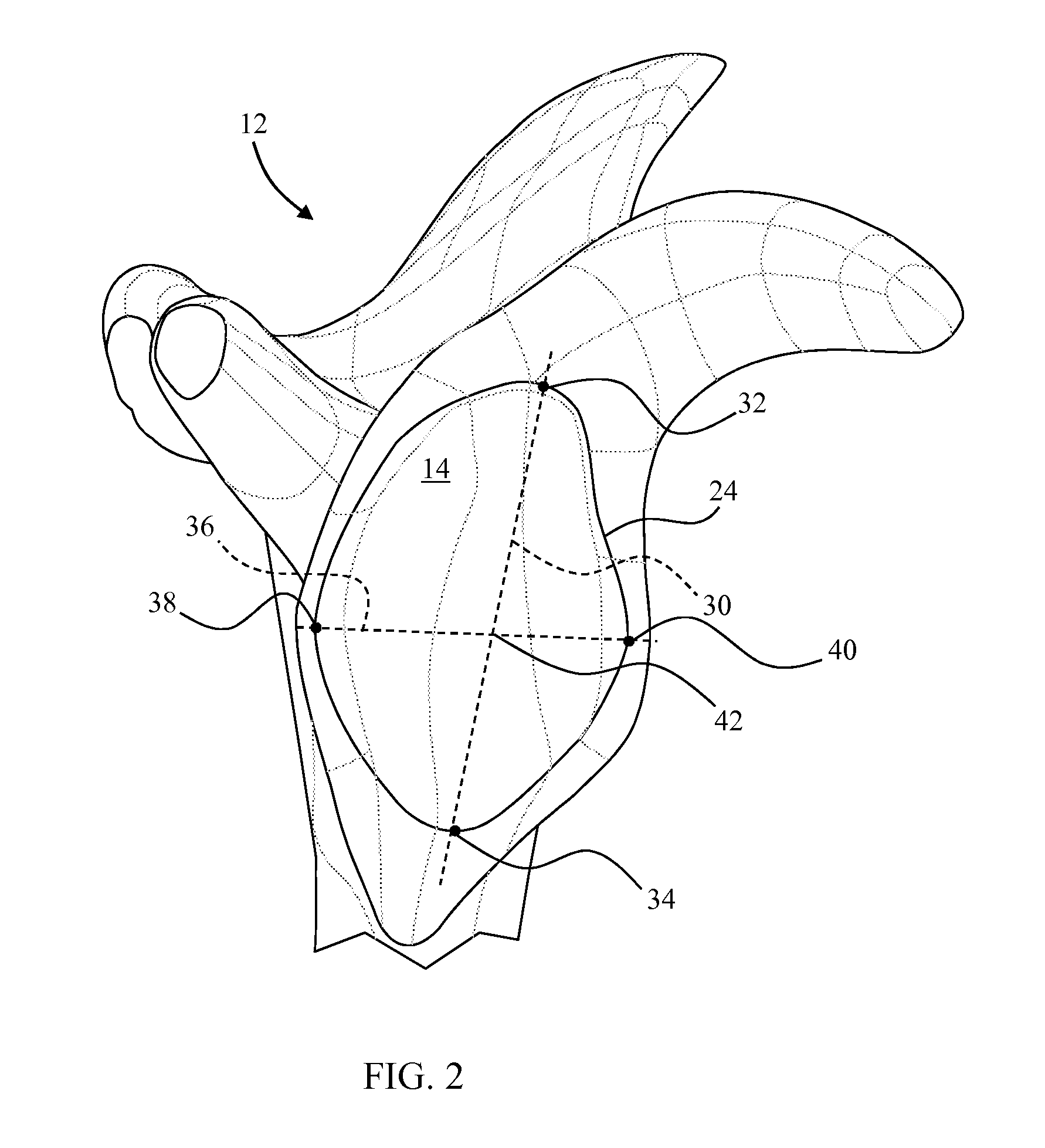
Glenoid component with an anatomically optimized keel
ActiveUS20070244564A1Improve stabilityHigh strengthJoint implantsShoulder jointsKeelBase of the sacrum
A glenoid component for a shoulder prosthesis adapted to be mounted in a glenoid cavity of a shoulder. An elongated keel adapted to engage with the glenoid cavity is attached to the internal surface of the base. The keel extends along a longitudinal axis of the base. The keel includes various configurations of transverse members extending away from the longitudinal axis. The surgeon selects a glenoid component with a keel and transverse member configuration that is anatomically optimized for the patient. Glenoid components properly optimized provide mechanical strength and stability superior to prior art devices. The glenoid components disclosed herein are particularly well suited for use in an anatomical total shoulder prosthesis, but may are also suited to partial shoulder prostheses and reverse shoulder prostheses.
Owner:TORNIER SA SAINT ISMIER
Glenoid component for shoulder arthroplasty
A glenoid component apparatus for shoulder arthroplasty includes a bearing portion and a stem portion connected to the bearing portion. The stem portion is modeled from a normalized glenoid vault morphology. A method for making a glenoid component for shoulder arthroplasty includes obtaining a model of a normalized glenoid vault morphology and producing a stem portion of the glenoid component based on the model.
Owner:DEPUY PROD INC
Total shoulder prosthesis or inverted type
InactiveUS20050278033A1High densityEasy to moveJoint implantsShoulder jointsArticular surfacesArticular surface
This total shoulder prosthesis comprises a glenoidal component, defining a substantially hemispherical convex articular surface and of which the centre of curvature is located in the glenoid cavity or in the immediate vicinity thereof, and a humeral component defining a concave articular surface adapted to cooperate with the convex articular surface of the glenoidal component. The concave humeral articular surface is defined by a metallic part of the humeral component, this avoiding having to resort to a part made of plastics material which would increase the space requirement of the prosthesis.
Owner:TORNIER SA SAINT ISMIER
Method for securing a glenoid component to a scapula
A medical procedure includes providing a prosthesis which includes (i) a body portion having a rear surface and a bearing surface, and (ii) an anchor peg including a shaft extending from the rear surface of the body portion, wherein the anchor peg includes a plurality of outwardly extending fins secured to the shaft, and further wherein the plurality of outwardly extending fins each possesses a first diameter. The medical procedure further includes creating an anchor hole in a natural bone, the anchor hole possessing a second diameter which is less than the first diameter. In addition, the method procedure includes positioning the anchor peg within the anchor hole so that each of the plurality of outwardly extending fins deforms so as to possess (i) a concave side which faces an open end of the anchor hole, and (ii) a convex side that faces a closed end of the anchor hole.
Owner:DEPUY PROD INC
Hybrid glenoid for shoulder arthroplasty
Glenoid prosthesis with a hybrid design (combining both peg and keel designs), and surgical methods for reconstitution of a shoulder joint. The hybrid design combines both peg and keel components into one device. The hybrid glenoid component of a shoulder prosthesis includes an oval body having a concave lateral articulating surface and an opposing convex medial surface. The medial surface is provided with a plurality of pegs and a single inferior protrusion. The pegs are used to attain both a superior location as well as a central location. The inferior protrusion provides stability in the superior-to-inferior translation of the humeral head, as well as rotational articulation of the same.
Owner:ARTHREX
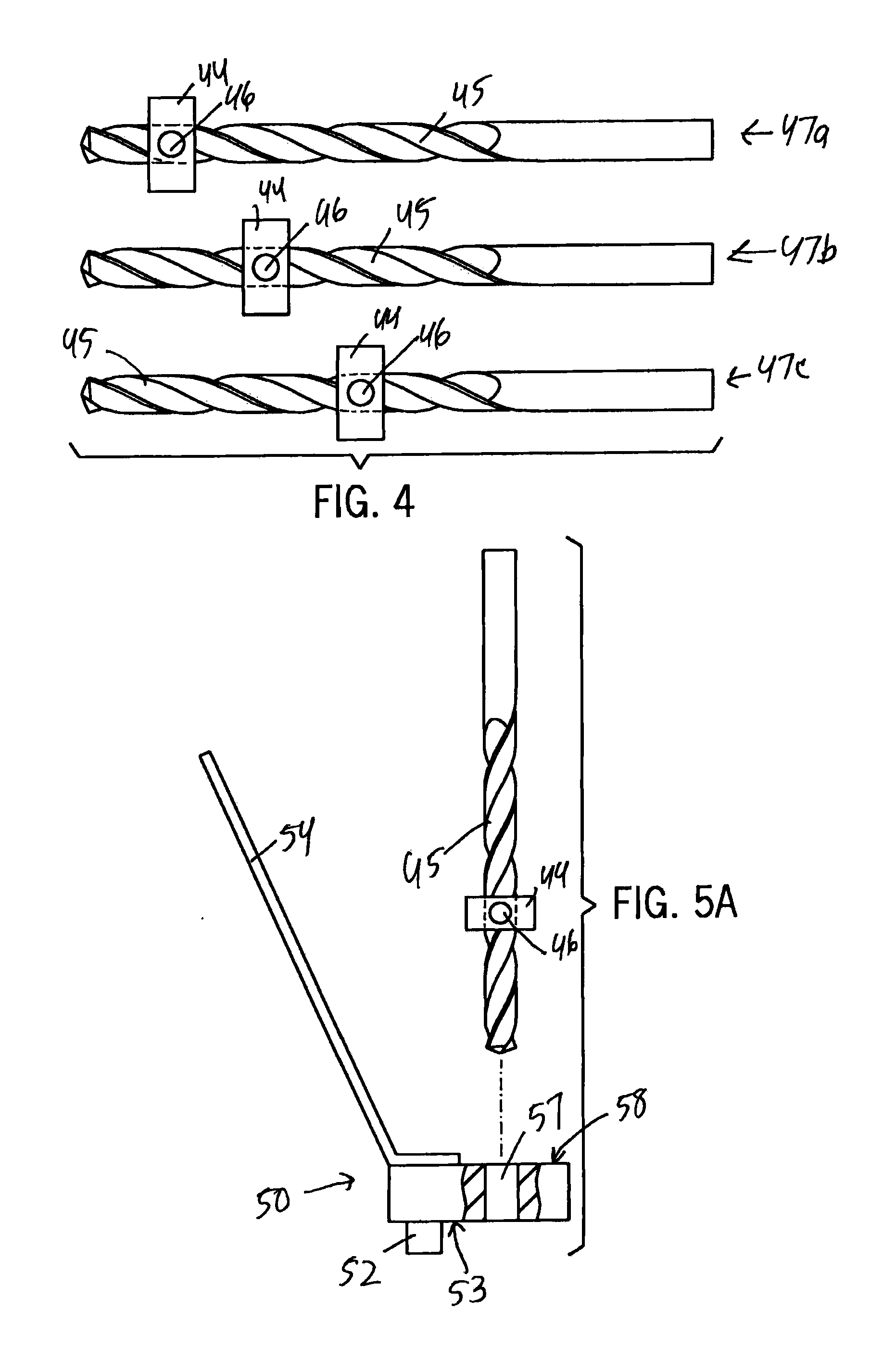
Combination Reamer/Drill Bit for Shoulder Arthoplasty
An instrumentation kit for use in preparing a shoulder to receive a glenoid component in one embodiment includes at least one combination device, the combination device including a boring section configured to form a first bore in a glenoid, a drive section operably connected to the boring section, a reaming section positioned proximally from the boring section and operably connected to the drive section, the reaming section configured to ream a portion of the glenoid, and at least one drill guide configured to guide a drill bit and positioned so as to guide the drill bit to form a second bore at a location spaced apart from the first bore, wherein the reaming section and the boring section are positioned with respect to each other such that the boring section forms the first bore and the reaming section simultaneously reams a portion of the glenoid adjacent to the first bore.
Owner:DEPUY SYNTHES PROD INC
Glenoid implant with synthetic labrum
A prosthetic glenoid component for replicating the behavior of a patient's natural glenoid includes a rim that replicates the patient's natural glenoid labrum.
Owner:ZIMMER INC
Revision glenoid device and method
A method of implanting a revision glenoid component in one embodiment includes accessing a previously implanted glenoid component in a scapula, removing the previously implanted glenoid component, identifying an inferior glenoid circle center of the scapula, preparing a glenoid fossa of the scapula to receive a revision glenoid component, selecting a revision glenoid component, and implanting the selected revision glenoid component based upon the identified inferior glenoid circle center in the prepared glenoid fossa.
Owner:DEPUY SYNTHES PROD INC
Glenoid component with an anatomically optimized keel
Owner:TORNIER SA SAINT ISMIER
Method and apparatus for shoulder arthroplasty
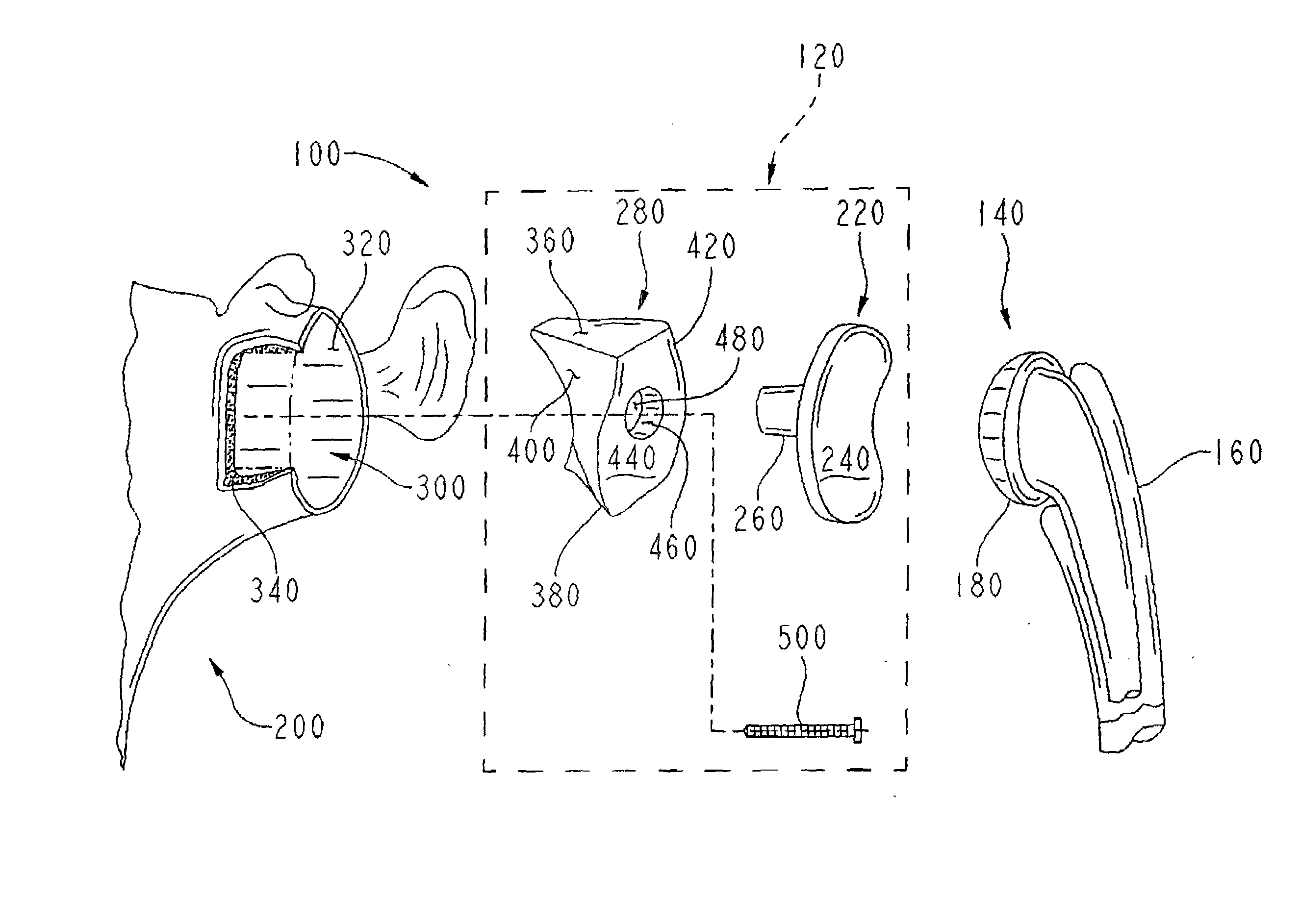
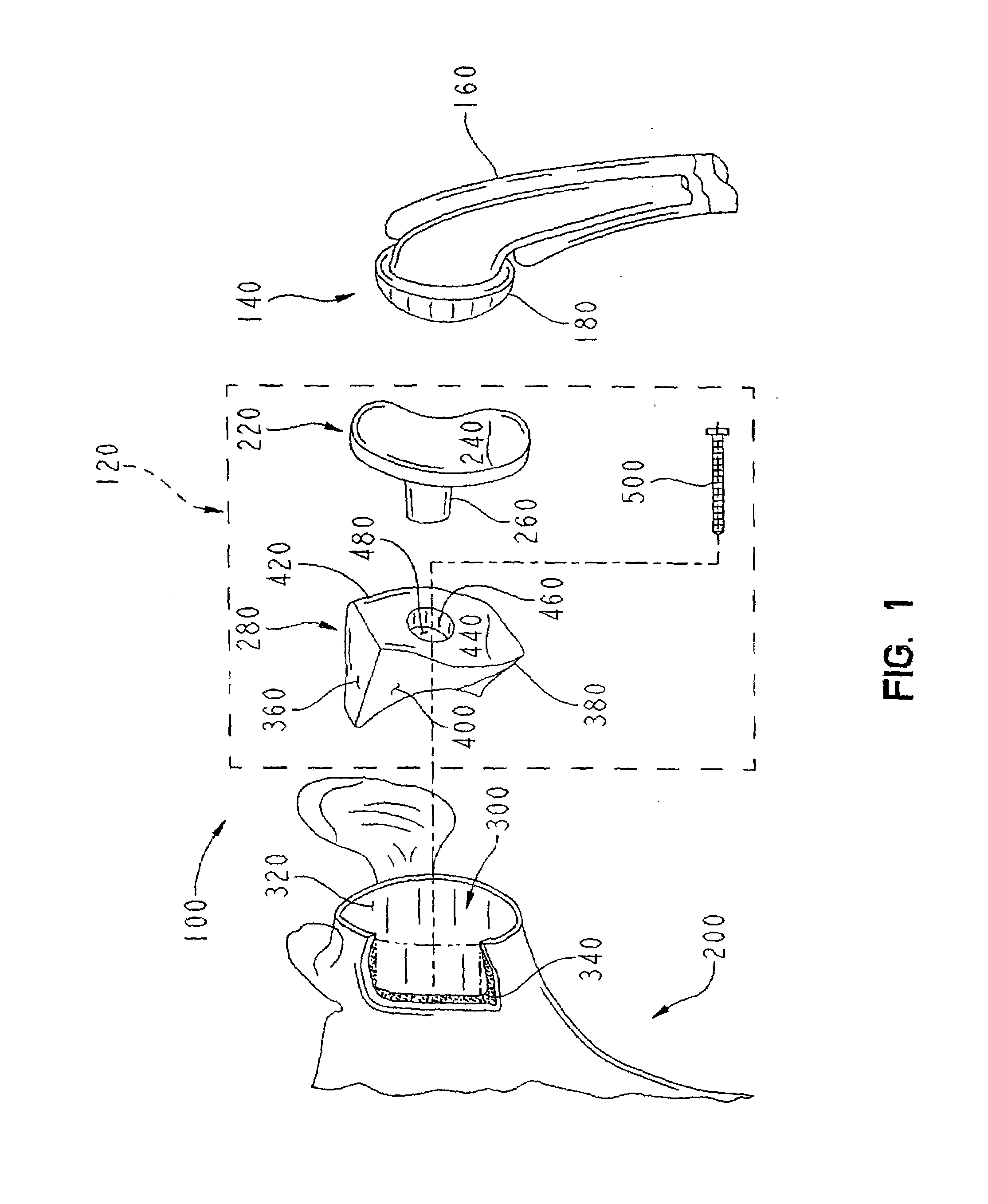
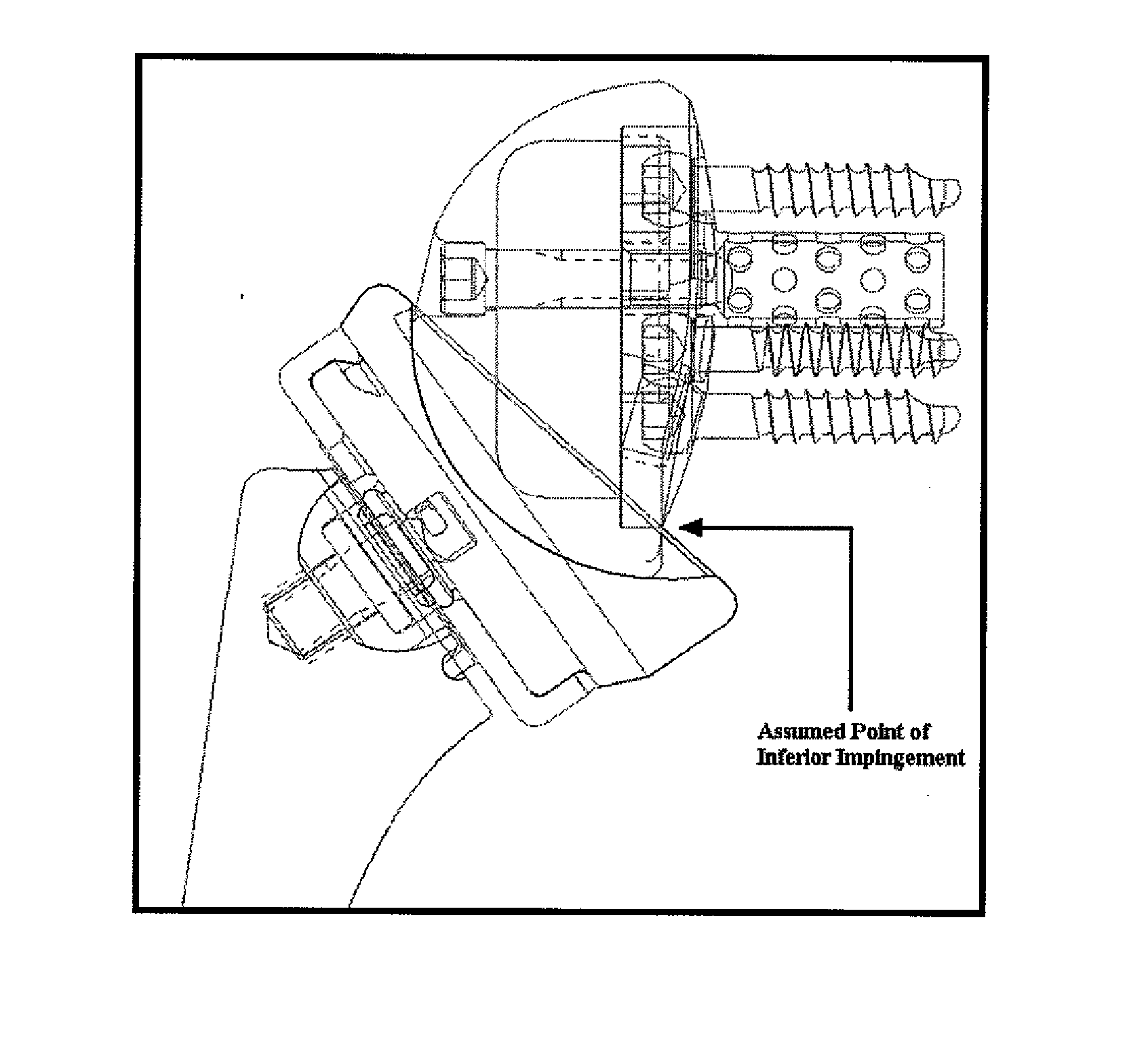
A prosthetic shoulder implant includes a humeral component having an anchor and a head, and a glenoid component. The glenoid component is defined by a body having an outer surface including one or more tissue and bone in-growing material areas, a concave inner surface for accepting the head of the humeral component, and defining a stop portion which prevents the head of the humeral component from moving upwardly into contact with the acromion or clavicle. A method of total shoulder arthroplasty includes locating the humeral and glenoid components so that the glenoid component prevents movement of the humeral component into contact with the acromion or clavicle, and such that the glenoid component is permanently anchored to the patient by tissue and bone ingrowth into the outer surface of the component.
Owner:SWANSON TODD V
Device and Method for Retroversion Correction for Shoulder Arthroplasty
A kit for implanting a glenoid component in one embodiment includes a retroversion glenoid component including a generally flat glenoid bone contacting surface defining a first plane, a first offset peg extending away from the glenoid bone contacting surface, a concave articulating surface, an upper surface extending about the concave articulating surface and generally opposite to the glenoid bone contacting surface, the upper surface defining a second plane, the second plane angled with respect to the first plane, and a drill guide configured to guide a drill in forming a bore in a scapula to receive the first offset peg.
Owner:DEPUY SYNTHES PROD INC
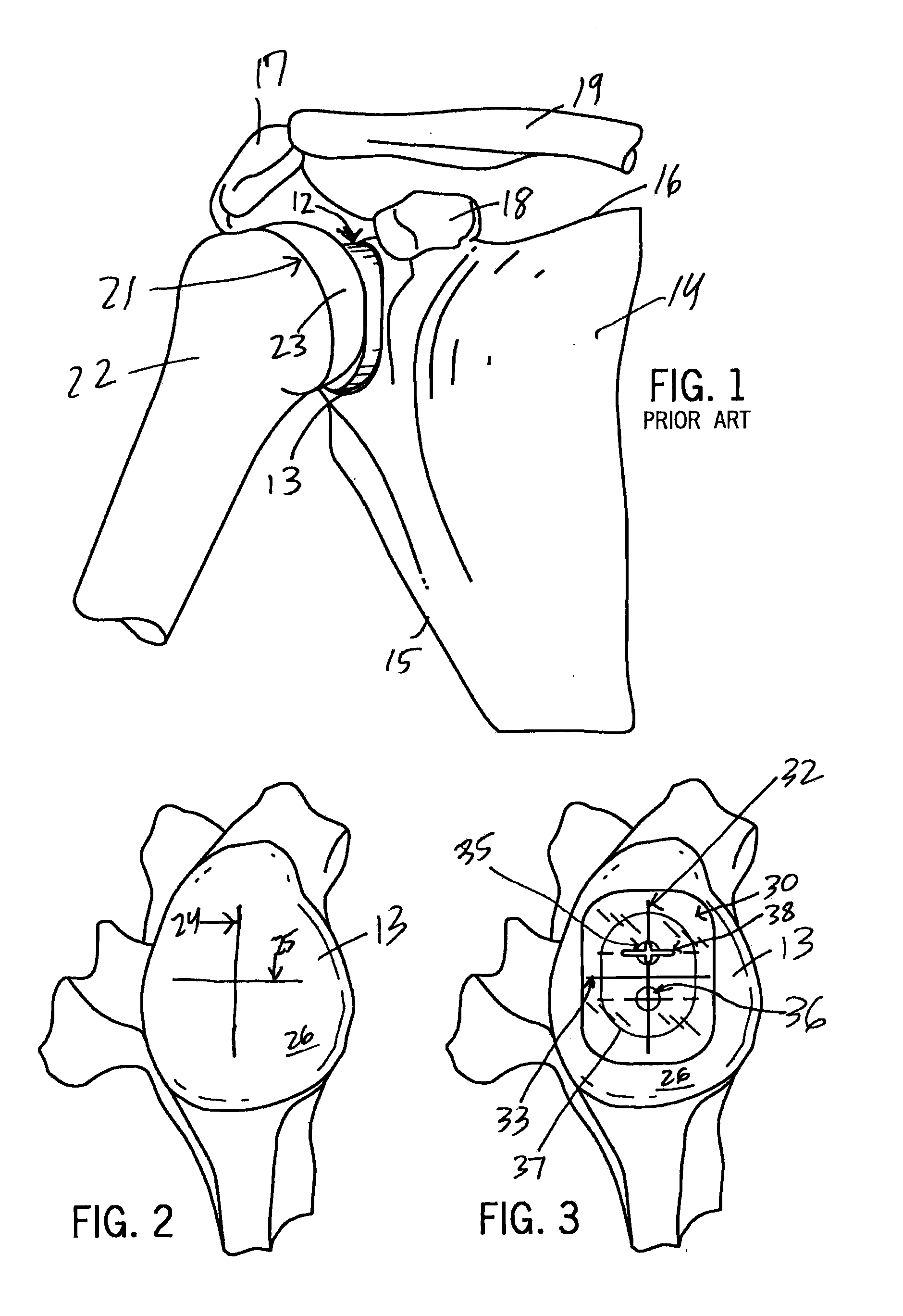
Circular glenoid method for shoulder arthroplasty
A method of shoulder arthroplasty in one embodiment includes accessing a scapula, identifying an inferior glenoid circle center of the scapula, preparing a glenoid fossa of the scapula to receive a prosthesis, selecting a glenoid component, and implanting the selected glenoid component based upon the identified inferior glenoid circle center in the prepared glenoid fossa.
Owner:DEPUY SYNTHES PROD INC
Glenoid component installation procedure and tooling for shoulder arthroplasty
InactiveUS20160143749A1Impart stabilityLongevityDiagnosticsJoint implantsArticular surfacesPilot hole
A method for implanting a glenoid component during shoulder arthroplasty is disclosed. In the method, a template is placed against a surface of a glenoid cavity of a scapula, and a bone marking tool is inserted through a cut-out guide in the template. The bone marking tool is used to create a bone mark on the surface of the glenoid cavity. A drill is aligned with the bone mark and a first pilot hole is drilled in the surface of the glenoid cavity. A locating pin of a first drill jig is placed in the first pilot hole, and a drill is inserted through a guide hole of the first drill jig to drill a second pilot hole in the surface of the glenoid cavity. Bone material is removed between the first pilot hole and the second pilot hole to create a slot in the surface of the glenoid cavity between the first pilot hole and the second pilot hole. A keel of a glenoid component is positioned in the slot, and the glenoid component is secured in the scapula. A kit is also provided for use with the method. The kit includes a glenoid component including a body having a first articulating surface and a second surface opposite the first articulating surface. The first articulating surface is dimensioned for engaging a head of a humerus or a humeral implant. The second surface is dimensioned for being secured to a scapula. The glenoid component further includes a keel extending away from the second surface. The kit also includes a transparent template having a cut-out guide dimensioned to receive a bone marking tool. The template preferably has an indication of a centerline of the template. The kit also includes a first drill jig having a locating pin and a guide hole dimensioned to receive a drill bit, and a cutting tool having a follower pin and a cutting surface.
Owner:THE GENERAL HOSPITAL CORP
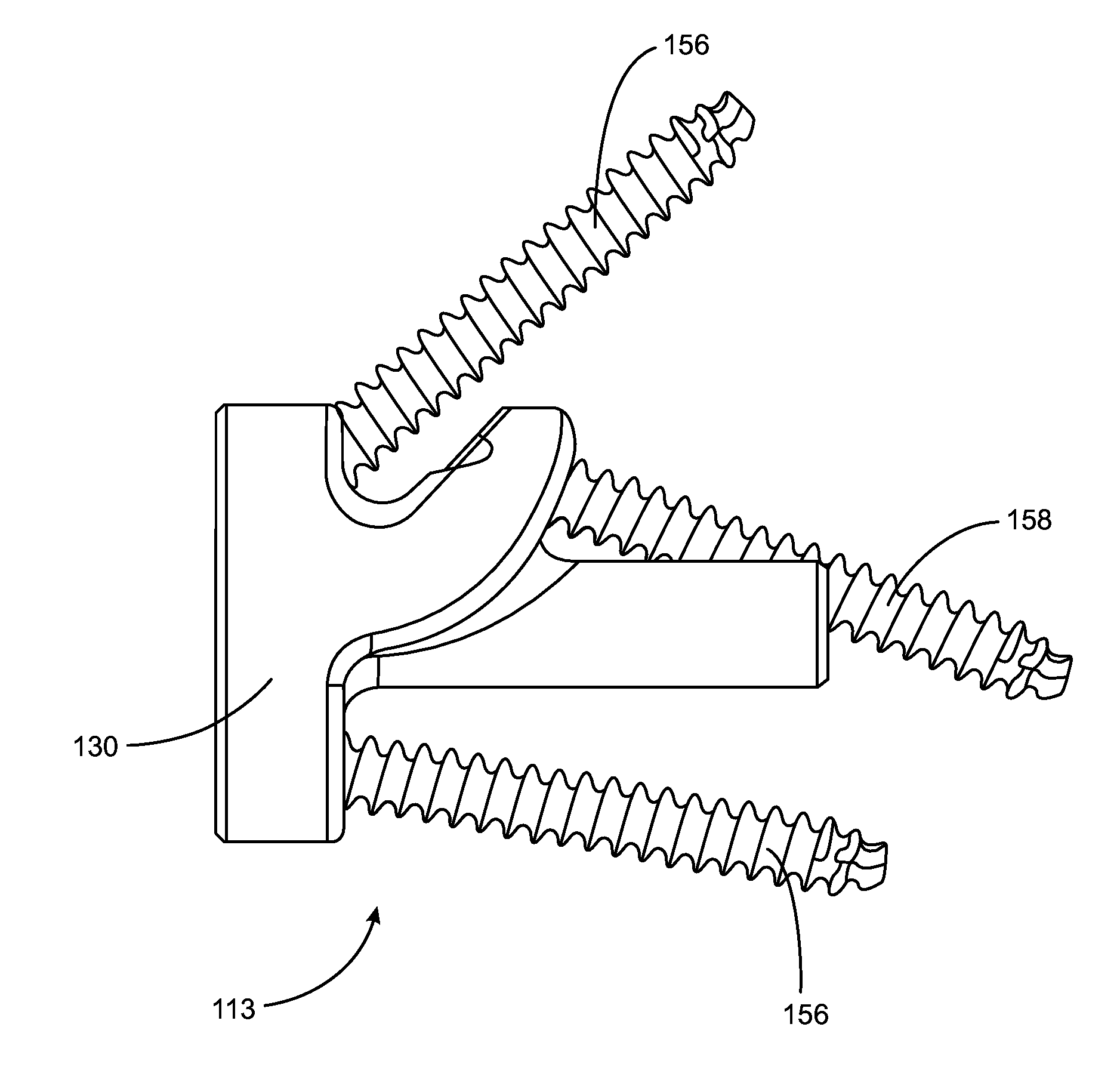
Rotatable reverse metaglene
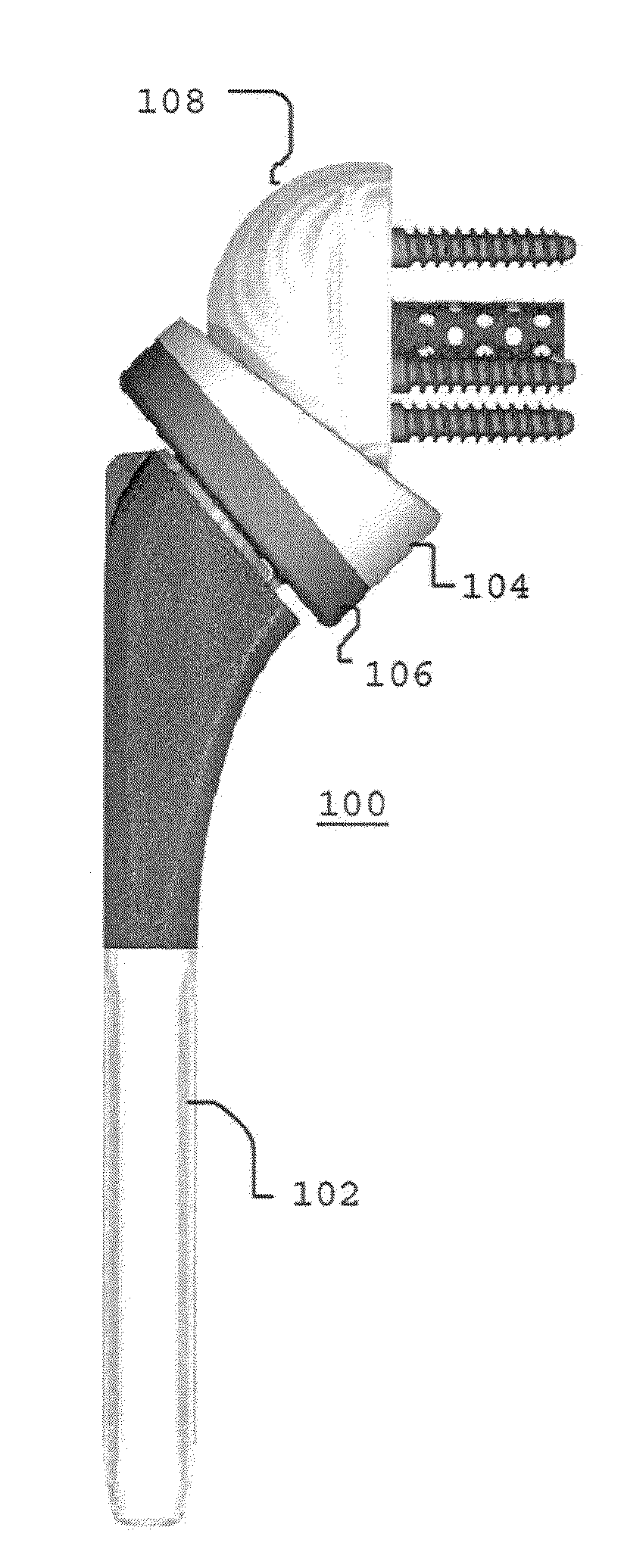
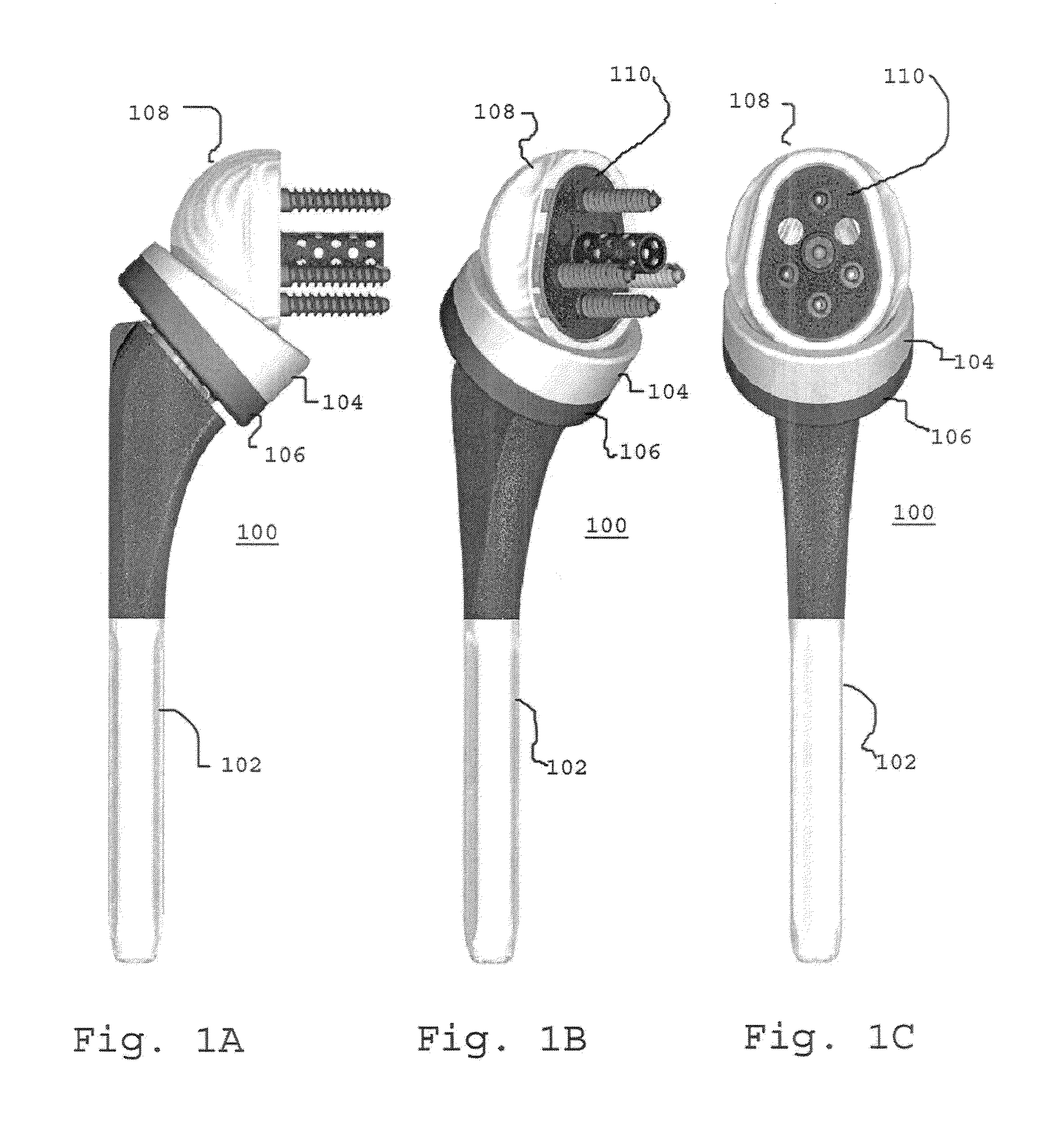
A metaglene assembly for use in a shoulder prosthesis includes a metaglene body, an augment, a void, at least one fastener hole, and at least one fastener. The metaglene body has a lateral, prosthesis-facing side, and a medial, bone-facing side. The augment extends medially from the medial, bone-facing side of the metaglene body. The void is defined by a portion of the medial, bone-facing side of the metaglene body and a lateral portion of the augment. The fastener hole extends from the void through the augment. The fastener is configured to extend within the fastener hole.
Owner:DEPUY SYNTHES PROD INC
Circular glenoid method for shoulder arthroplasty
A method of shoulder arthroplasty in one embodiment includes accessing a scapula, identifying an inferior glenoid circle center of the scapula, preparing a glenoid fossa of the scapula to receive a prosthesis, selecting a glenoid component, and implanting the selected glenoid component based upon the identified inferior glenoid circle center in the prepared glenoid fossa.
Owner:DEPUY SYNTHES PROD INC
Tamp for concave resurfacing prosthesis
InactiveUS20080109000A1Suitable for useDiagnosticsBone implantSacroiliac jointBiomedical engineering
A tamp for use in forming grafting material into a cavity in the glenoid vault to prepare the vault for a glenoid component for performing shoulder arthroplasty is provided. The tamp includes a body and a forming portion. The forming portion extends in a first direction from the body. The forming portion including a surface thereof shaped to receive the glenoid component.
Owner:DEPUY PROD INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com