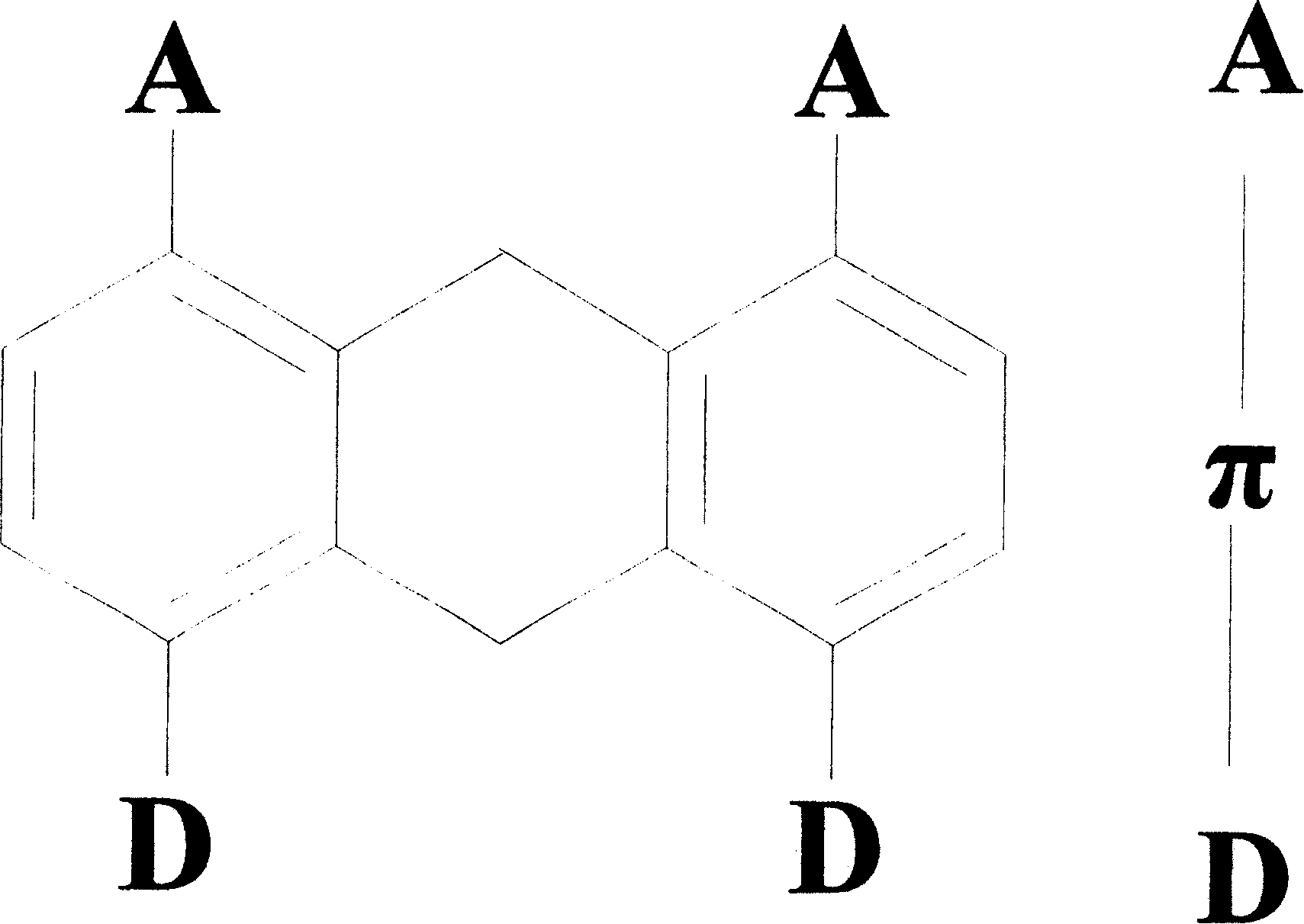
9,10-dihydroanthracene chromophore compound and its preparing process
A technology of dihydroanthracene and compound is applied in the field of chromophore compound and its preparation, which can solve the problems such as decrease in transmittance, and achieve the effects of good transmittance and large second-order nonlinear coefficient.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Preparation of 4,5-dimethoxy-1,8-diformyl-9,10-dihydroanthracene (II) and its physical and chemical parameters.
[0045] In a 250mL four-necked bottle, add 30mL of chloroform solution containing 6-7mmol 1,1-dichloromethyl ether, pass N 2Protect, heat up to 40°C with electromagnetic stirring, and dropwise add 30 mL of chloroform solution containing 1 g (4 mmol) of compound I and 30 mL of chloroform solution containing 2.0 g of titanium tetrachloride at the same time using two dropping funnels, and continue stirring for 2 hours after the addition is complete. Cool to room temperature, pour the reaction solution into 500mL water, stir, filter, extract the liquid phase with chloroform, wash with water until neutral, separate the layers, dry with anhydrous sodium sulfate for 0.5 hours, filter, evaporate the solvent under reduced pressure, and wash with chloroform / Recrystallization from cyclohexane gave 1.34 g of compound II. Yield: 64%, melting point: 248-250°C ...
Embodiment 2
[0047] Embodiment 2: the preparation method of compound III1a, III1b and its physical and chemical parameters:
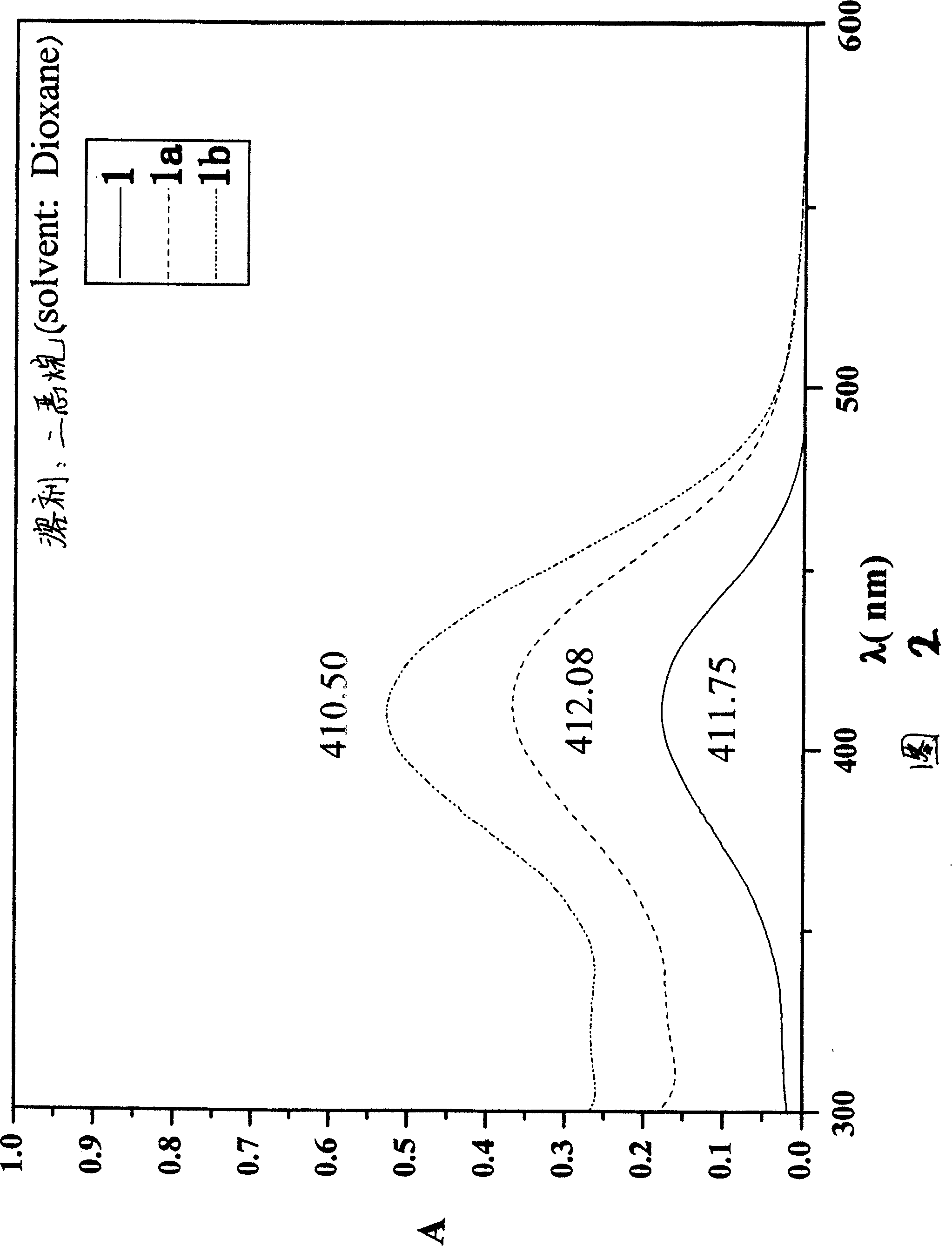
[0048] Add 0.3g (1.0mmol) of compound II into a 50mL three-neck flask filled with 10mL of absolute ethanol, pass through nitrogen protection, stir electromagnetically, and heat to reflux. Add 0.4g (2.90mmol) of compound 1A, add dropwise a 10mL absolute ethanol solution containing 5 drops of hexahydropyridine and 10 drops of acetic acid, and heat to reflux for 50 hours. Cool to room temperature, add 60 mL of chloroform, wash with water until neutral, and dry over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the resulting residue was separated by silica gel column chromatography (ethyl acetate:petroleum ether=1:10) to obtain a red solid, which was recrystallized from acetone / petroleum ether to obtain 0.066g / 0.034g of compound III1a / III1b, both It is a red crystal.
[0049] (III1a) productive rate: 14.4%; Melting point: 250~252 ℃; ...
Embodiment 3
[0051] Embodiment 3: the preparation method of compound III2a, III2b and its physical and chemical parameters:
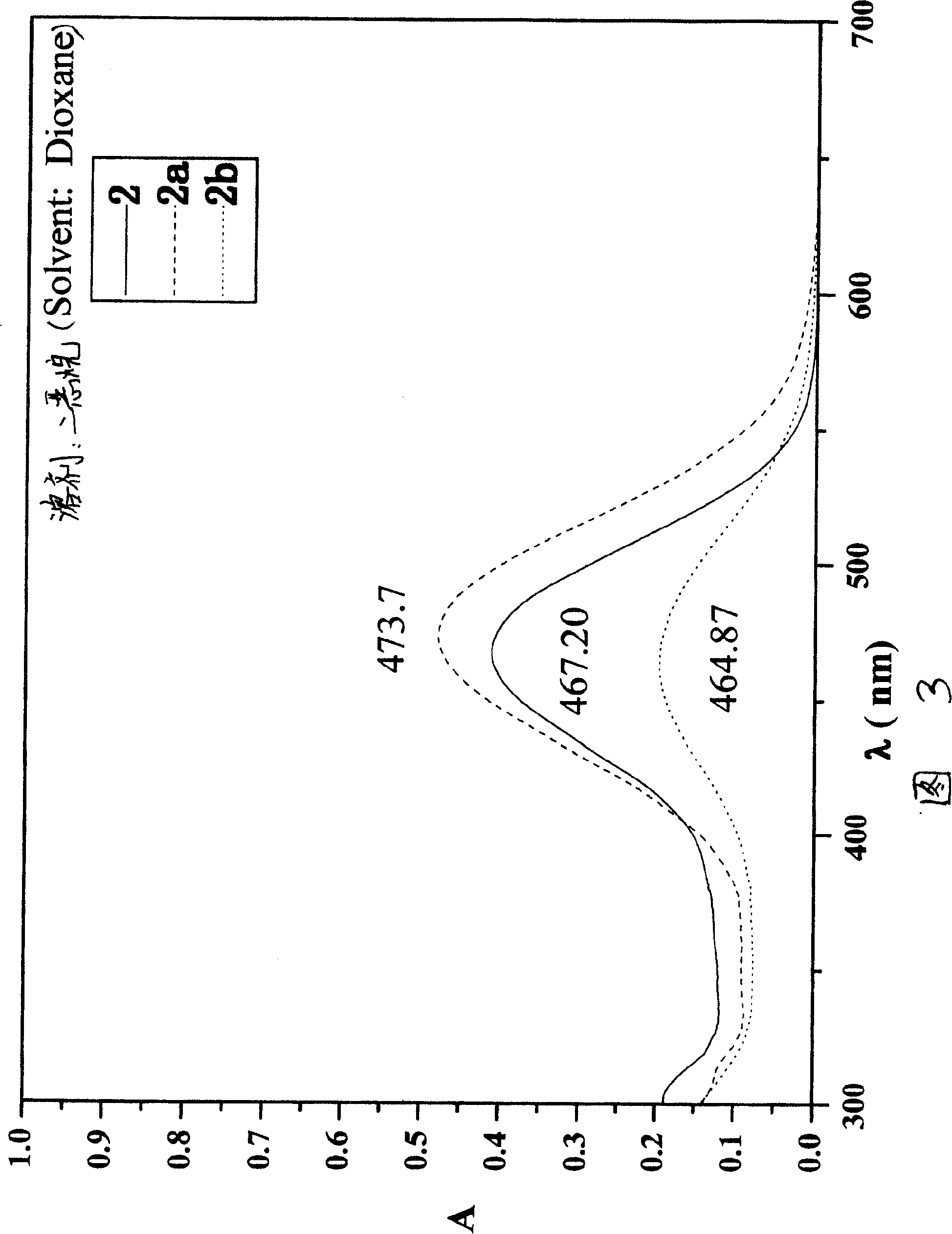
[0052] In a 50mL three-necked flask, heat 0.3g (1.0mmol) of compound II in 20mL of absolute ethanol to reflux, after dissolving, add 0.64g (2mmol) of compound 2A, and after a few minutes, add dropwise 10mL containing 8 drops of hexahydro Pyridine and 16 drops of acetic acid in absolute ethanol. Heat to reflux for 60 hours. Cool to room temperature, add 75 mL of chloroform, wash with water until neutral, and dry over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the resulting residue was separated by column chromatography (ethyl acetate:petroleum ether=1:10 as eluent) to obtain a purple solid, which was recrystallized from acetone / petroleum ether to obtain 0.196g / 0.067g of compound III2a / III2b, all purple solids.
[0053] (III2a) Yield: 33.6%, melting point: 254-256°C. MS: m / e=599 [M + ]. 1 HNMR (300MHz, CDCl 3 ): δ(ppm)=10....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com