Process for preparing human leucocyte interleukin 24 by genetic engineering and it expressing carrier and engineering bacterium
A technology of interleukin and genetically engineered bacteria, which is applied in the field of genetic engineering preparation of human interleukin 24 and its expression vector and engineered bacteria, can solve the problems of no experimental research on biological activity verification, short-term effects, and application limitations. To achieve the effect of improving the refolding effect and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
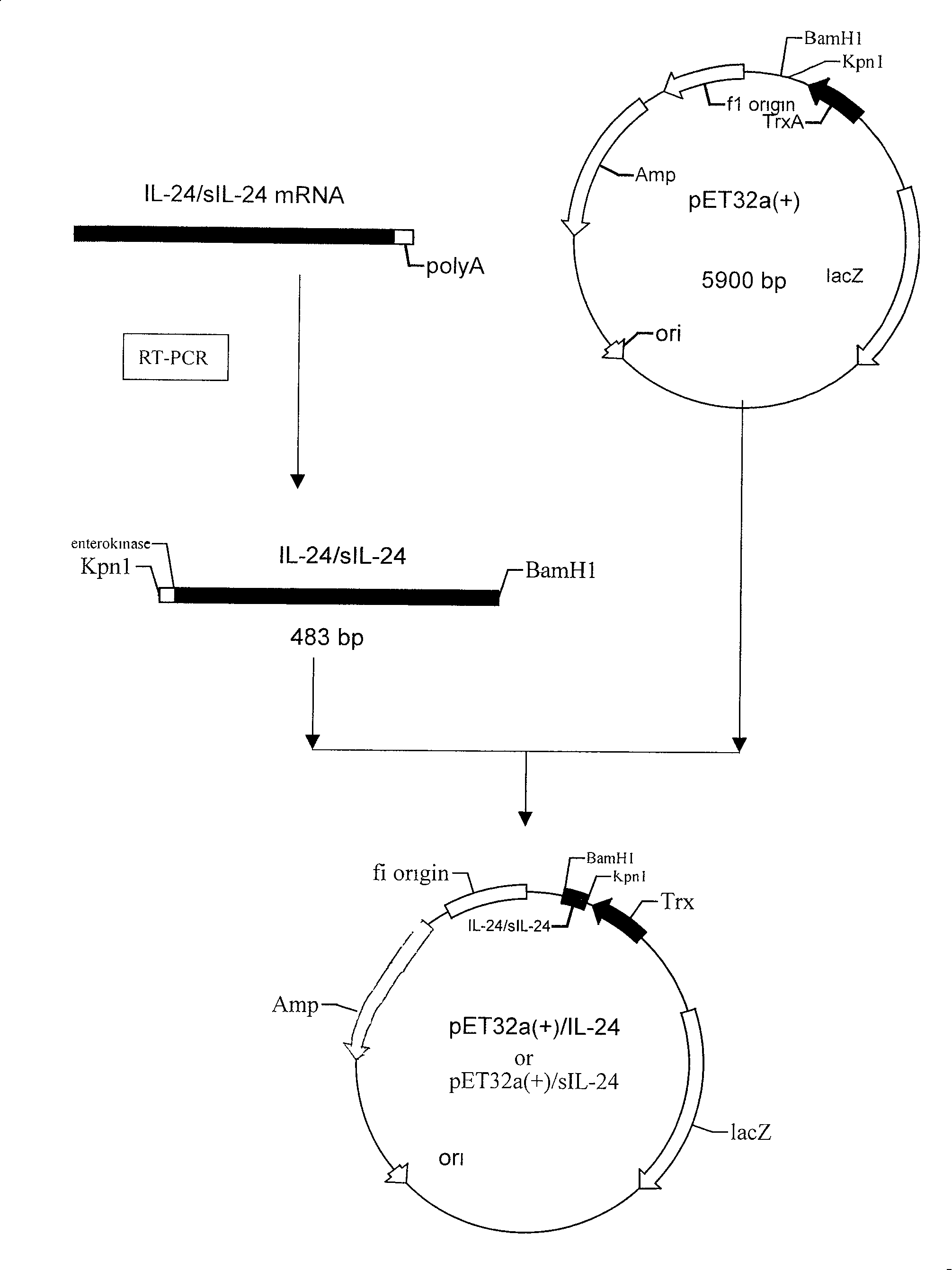
[0039] Cloning of the coding gene of embodiment 1 human IL-24:
[0040] 1. Extraction of total RNA: Take 5 mL each of anticoagulated blood from healthy people, and collect the buffy coat with lymphocyte separation medium. Add RPMI1640 complete medium and ConA with a total concentration of 25mg / L, 37°C, 50mL / L CO 2 After incubation for 48 h, the cells were collected by centrifugation. RNA extraction kit was used to extract total RNA.
[0041] 2. RT-PCR: using total mRNA as a template, reverse transcription to obtain IL-24cDNA, the conditions are 30°C for 10s, 50°C for 30s, 99°C for 5s, 5°C for 5s, 1 cycle.
[0042] 3. PCR amplification of the target gene: Design a pair of primers according to the IL-24 sequence published by GenBank. The primers are:
[0043] P1 5'- GGTACC GACGACGACGACAAGGCCCAGGGCCAAGAATT(Kpn1)
[0044] P2 5'- GGATCC TTACAGAGCTTGTAGAATTTCTG (BamHl)
[0045] In order to avoid the introduction of two redundant amino acids Ala and Met at the N-terminal, th...
Embodiment 2
[0065] Example 2 Construction of recombinant expression plasmid pET32a(+)-IL-24 or pET32a(+)-hsIL-24 and construction and screening of high-efficiency expression engineering bacteria
[0066] 1. Construction of recombinant plasmids
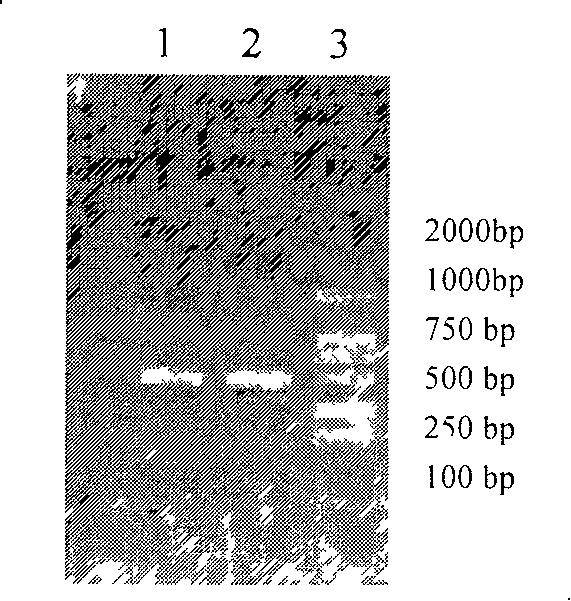
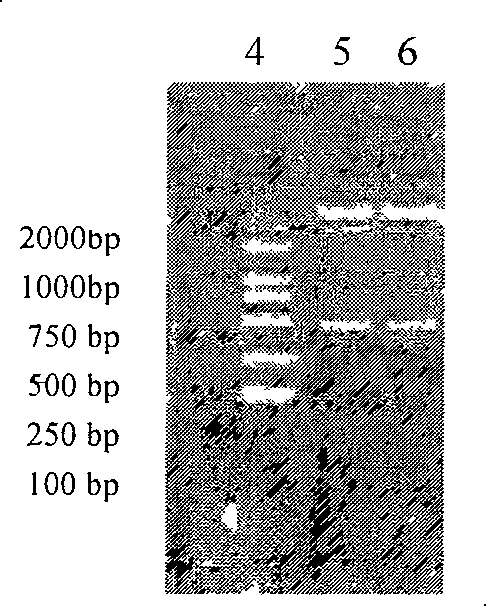
[0067] The IL-24 or hsIL-24 gene amplification (PCR) product was subjected to 1.0% agarose electrophoresis, gel recovery and purification, and then ligated with the vector pMD-18T, transformed into Escherichia coli DH5α, extracted the plasmid, digested with Kpn1 and BamH1, 1.0% Identification by agarose gel electrophoresis.
[0068] Digest the pMD-18T vector and pET-32a(+) containing the IL-24 or hsIL-24 target gene with Kpn1 and BamH1, and the digested products are subjected to 1.0% agarose electrophoresis, and the target fragment gel is recovered and purified. Ligation, transformation of Escherichia coli DH5α, extraction of plasmids, digestion of Kpn1 and BamH1, and identification by 1.0% agarose gel electrophoresis. Enzyme digestion results a...
Embodiment 3
[0130] Fermentation of embodiment 3 gene recombinant bacteria
[0131] The fermentation process is as follows:
[0132] German B.Bron 10L fermenter was used. During the fermentation process, 10% of the seed bacteria were inoculated, 70% dissolved oxygen was maintained, the temperature was 37°C, and the pH was 7.0. When the A600 did not reach 2, no feed was added, and then every 0.5h. Feed once so that the final concentrations of glucose, tryptone and 8% yeast extract are 0.5%, 0.2%, and 0.2%, respectively. After the 4th feed, when the glucose concentration dropped to 0.1%, IPTG 500 μmol / L was added to induce the harvest for 4 hours.
[0133] The fermentation process is based on the batch culture controlled by cascading dissolved oxygen, and feeding is added.
[0134] The medium used in the fermentation process is the improved M9CAA medium, on the basis of M9-CAA, 0.6% yeast extract and 2mg / L ZnCl are added 2 4H 2 O, 2mg / LCoCl 2 4H 2 O, 4mg / L FeSO 4 ·16H 2 O, 5mg / L H 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com