Doxycyline Hyclate lipid gels and preparing method
A technology of doxycycline hydrochloride and body gel, which is applied in the directions of liposome delivery, tetracycline active ingredients, pharmaceutical formulations, etc. The effect of prolonging the action time and improving the antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
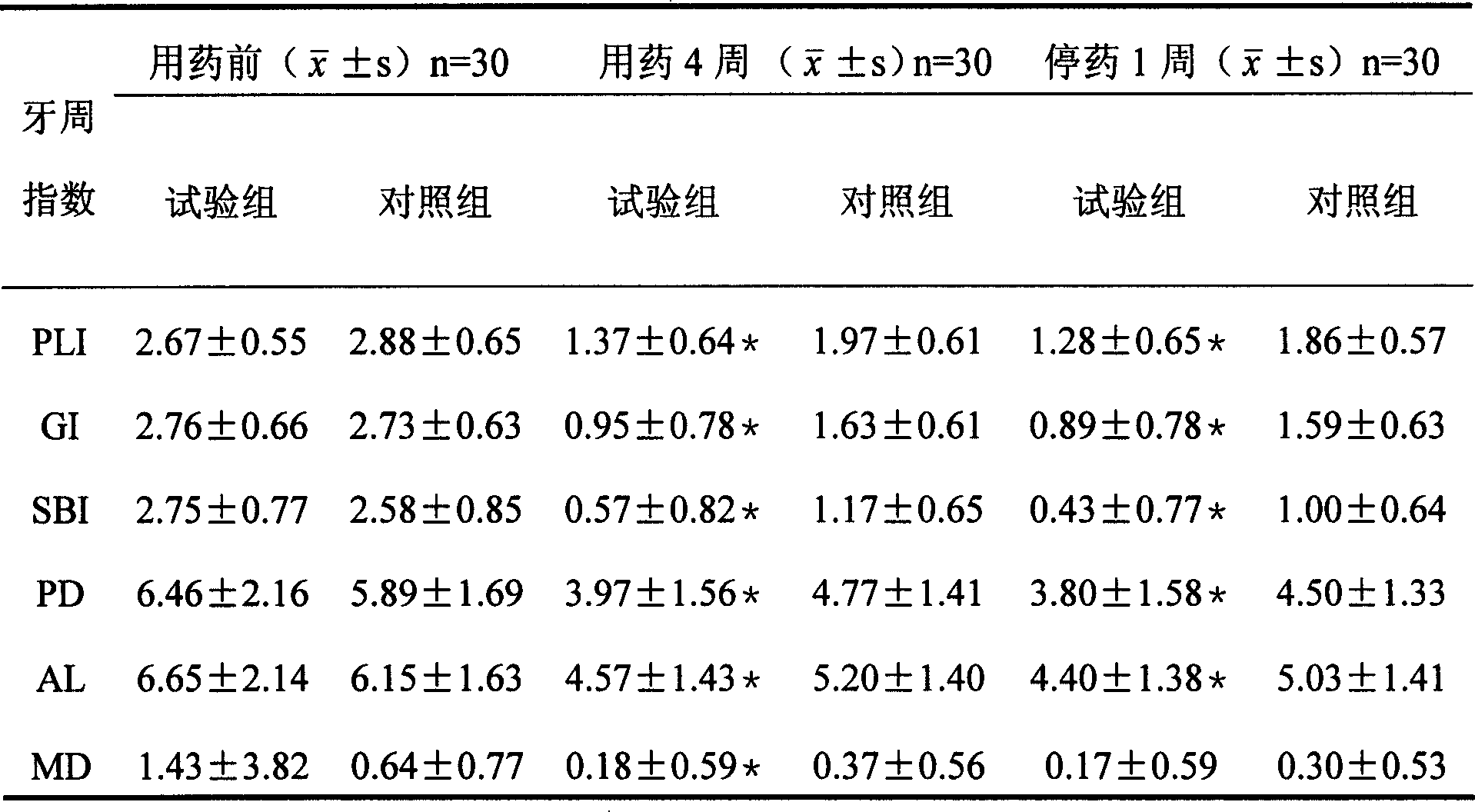
[0032] 500mg of soybean lecithin and 100mg of cholesterol were co-dissolved in 20ml of ethanol, and formed into a film by rotary evaporation in a water bath at 50°C; Phosphate buffer saline, and this solution is added in the film, oscillates to disperse and stir homogenizes one to two hours, obtain doxycycline hydrochloride liposome, add 0.1wt% sulfurous acid to doxycycline hydrochloride liposome Sodium hydrogen, stored in the refrigerator for later use.
[0033] The average particle size of the obtained doxycycline hydrochloride liposome is between 100-200 mm; the product is applied to the ultrafiltration centrifugation method to determine the encapsulation efficiency of more than 60%.
[0034] Swell 0.6 g of carbopol 934P with distilled water equivalent to 20 times the weight of the carbopol, add triethylamine dropwise to make the pH value about 9, and obtain the carbopol gel matrix, which is stored for later use.
[0035] Mix the doxycycline hydrochloride liposome and the ...
Embodiment 2
[0037] Dissolve 1000 mg of soybean lecithin and 100 mg of cholesterol in 30 ml of ether, and form a film by rotary evaporation in a water bath at 50 ° C. Dissolve 200 mg of doxycycline hydrochloride in 5 mL of phosphoric acid at pH = 7.3 containing 2% poloxamer Add the solution to the membrane, oscillate to disperse and homogenize with stirring for one to two hours to obtain doxycycline hydrochloride liposomes.
[0038] The average particle diameter of the obtained doxycycline hydrochloride liposome is between 100-200nm; the encapsulation efficiency of the product is determined to be over 70% by an ultrafiltration centrifugation method.
[0039] Swell 0.7 g of carbopol 971P with distilled water equivalent to 5 times the weight of the carbopol, add dropwise 1 mol / L sodium hydroxide to make the pH value about 11, and then obtain the carbopol gel matrix, which is stored for later use.
[0040] Mix the doxycycline hydrochloride liposome and the gel base at a ratio of 8:2 to obtain...
Embodiment 3
[0042] Dissolve 1500mg of egg yolk lecithin and 150mg of cholesterol in 20ml of ethanol, dissolve 300mg of doxycycline hydrochloride in 10mL of phosphate buffer containing 5% poloxamer at pH=6, pour the ethanol solution into the buffer solution , and the mixed solution was sheared with a high-speed shear to form colostrum, and the emulsion was evaporated under reduced pressure to obtain a doxycycline hydrochloride liposome suspension.
[0043] The average particle diameter of the obtained doxycycline hydrochloride liposome is within 100nm; the encapsulation efficiency of the product is determined to be 55% by an ultrafiltration centrifugation method.
[0044] Swell 1 g of carbopol 940 with distilled water equivalent to 10 times the weight of the carbopol, add dropwise sodium bicarbonate to make the pH value about 10, and obtain the carbopol gel matrix, which is then placed for later use.
[0045] Mix the doxycycline hydrochloride liposome and the gel base at a ratio of 5:5 to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com