Synthesis of organic free radical polyalcohol PTMA anode material of lithium secondary battery and uses of the same
A lithium secondary battery and positive electrode material technology, which is applied in the synthesis process of free radical polymer PTMA, can solve the problems of poor fast charge and discharge performance, capacity attenuation, and structural instability, and achieve biodegradability and capacity attenuation The effect of slowing down and eliminating potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
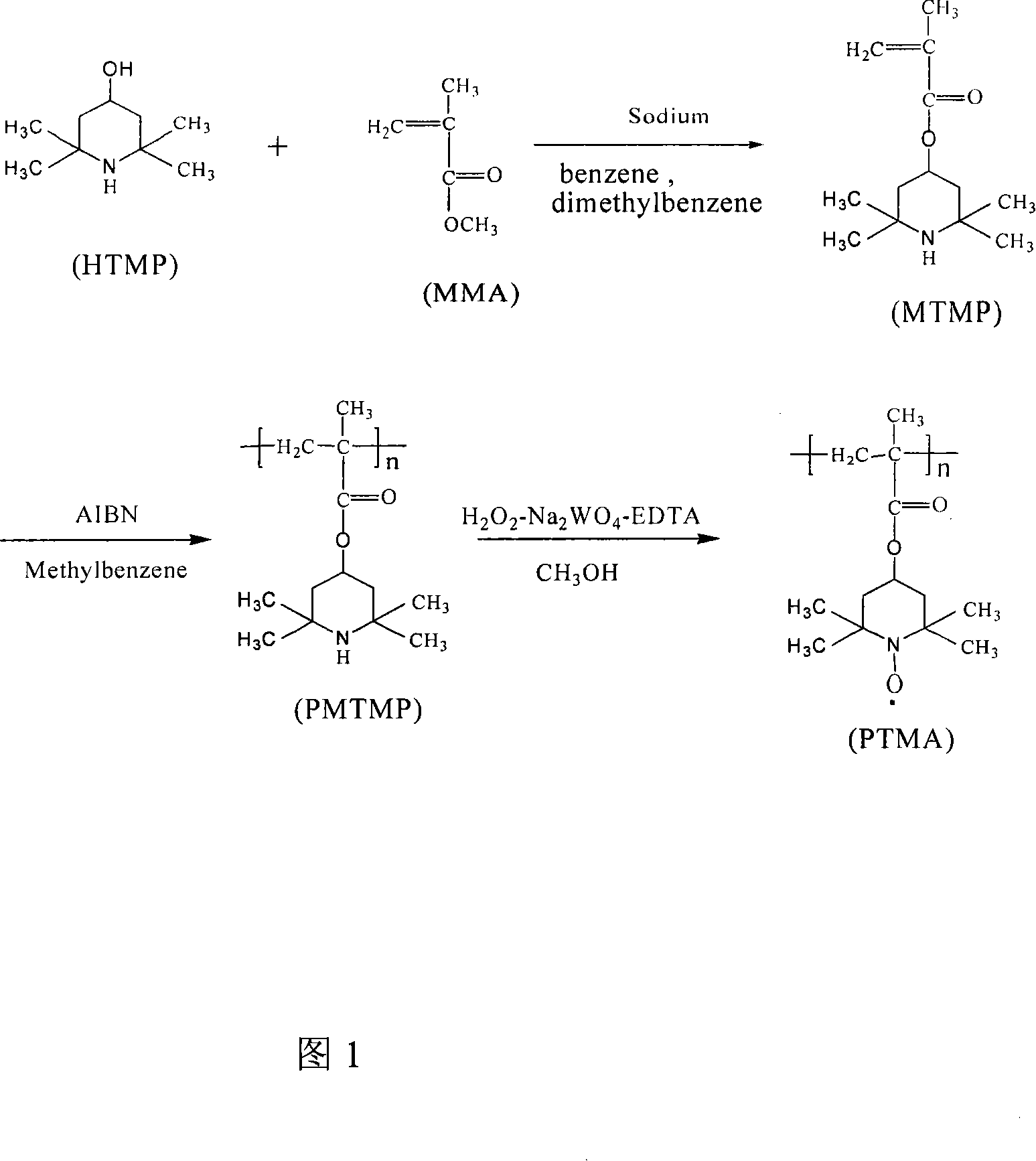
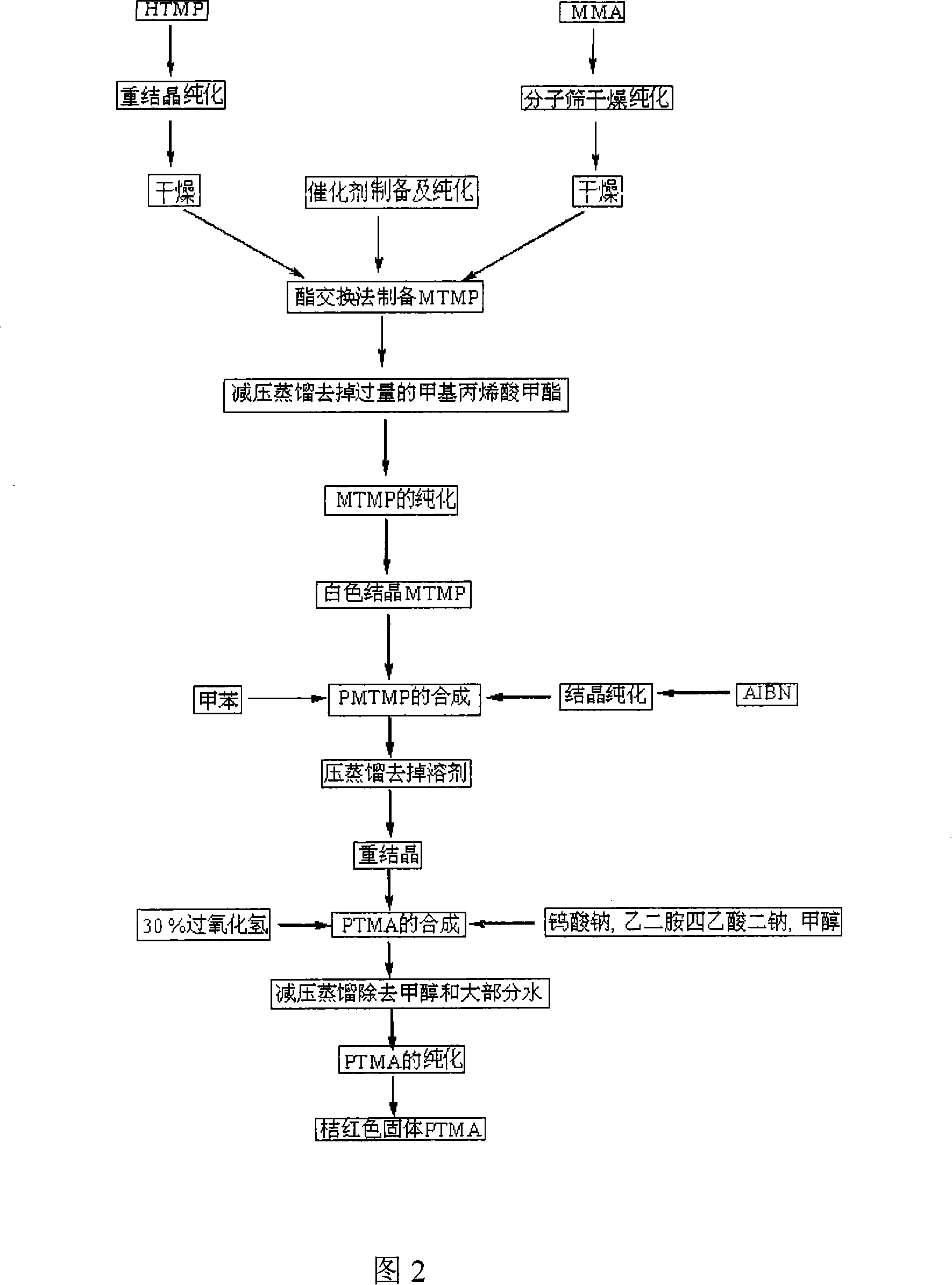
[0043] (1) Chemical pretreatment process of raw materials, 2,2,6,6-tetramethyl-4-hydroxypiperidine (HTMP for short), recrystallized from benzene-ethanol, m.p.128~129℃; methyl methacrylate Ester (referred to as MMA), methyl methacrylate is dried by molecular sieve and then distilled for use; metallic copper (99.9%); magnesium powder (active magnesium content 99.0%); azobisisobutyronitrile (referred to as AIBN) is purified by ethanol crystallization Use; solvent tetrahydrofuran and ether with CaCl 2 After drying, it was used by refluxing with sodium benzophenone.
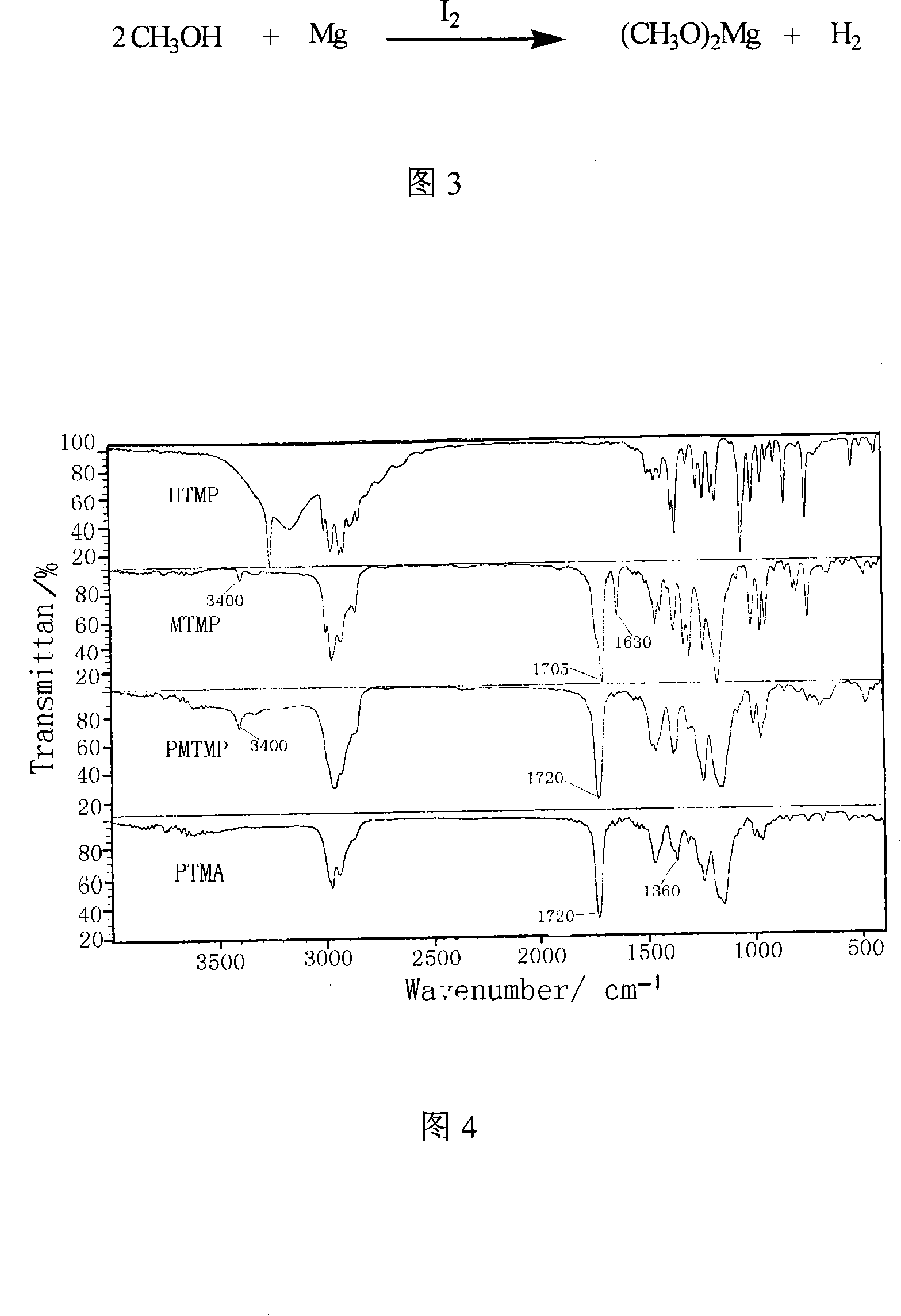
[0044] (2) The preparation process of the catalyst magnesium methoxide, adding magnesium powder and methanol in a reaction kettle with a stirrer, and then adding a small amount of iodine as an initiator. After 2 to 3 minutes of reaction at room temperature, the color of iodine faded and began to emit Hydrogen, after the reaction for 1 to 2 hours, no more hydrogen is released, the reaction solution becomes a white pas...
Embodiment 2
[0054] The PTMA obtained in Example 1 was used as an organic positive electrode material for a lithium secondary battery
[0055] (a) Assembly of the battery
[0056] The battery uses the PTMA aluminum foil electrode as the positive electrode and the metal lithium sheet as the negative electrode. The electrolyte is 1mol·L -1 LiPF 6 / EC-DMC (1:1 by volume). The assembly of the cells was carried out in an argon-filled glove box.
[0057] (b) Working principle of PTMA / lithium secondary battery
[0058] The working principle of PTMA / lithium secondary battery refers to its charging and discharging principle, which is the same as that of lithium secondary battery. According to the ESR spectra of oxidized PTMA (4.2V) and reduced PTMA (3V), as well as the charge-discharge and CV curves of PTMA, PTMA is at E a,p = The oxidation peak at 3.644V is that the O-N group of PTMA is oxidized and loses an electron to form a positive ion salt with the O=N group and the electrolyte anion, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
| Specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com