Multi-phase ion-electron mixing conductor powder material, manufacturing method and application thereof
A technology of mixing conductors and powder materials, applied in the direction of non-metallic conductors, circuits, electrical components, etc., can solve problems such as single, few, and impossible
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of complex ion-electronic mixed conductor powder material
[0030] According to the chemical formula BaCo x Fe y Nb z o 3-δ , where x=0.7, y=0.2, z=0.1; the dopant is ZrO 2 , its doping amount is 2.5%, 5%, 7.5% and 10% relative to the mass fraction of BCFN; the specific steps are as follows:
[0031] 1. Weigh 395g of BaCO respectively 3 , 116g of Co 2 o 3 , 32g of Fe 2 o 3 and 26.6 g of Nb 2 o 5 , mix each raw material with ethanol or water as a dispersant ball mill, mix evenly and dry, then bake at 1000°C-1150°C for 10-20 hours, and then dry by ball mill to get BaCo x Fe y Nb z o 3-δ doped powder;
[0032] 2. Mix 100g of BCFN powder obtained in step 1 with 2.5g, 5g, 7.5g and 10g of dopant ZrO 2 , after ball milling and mixing for 1-2 hours; mixed powder is obtained;
[0033] 3. Pressure-form the mixed powder obtained in step 2 into a diaphragm green body, heat up to 1150°C for 20 hours at a heating rate of 5°C / min, and sinter at ...
Embodiment 2
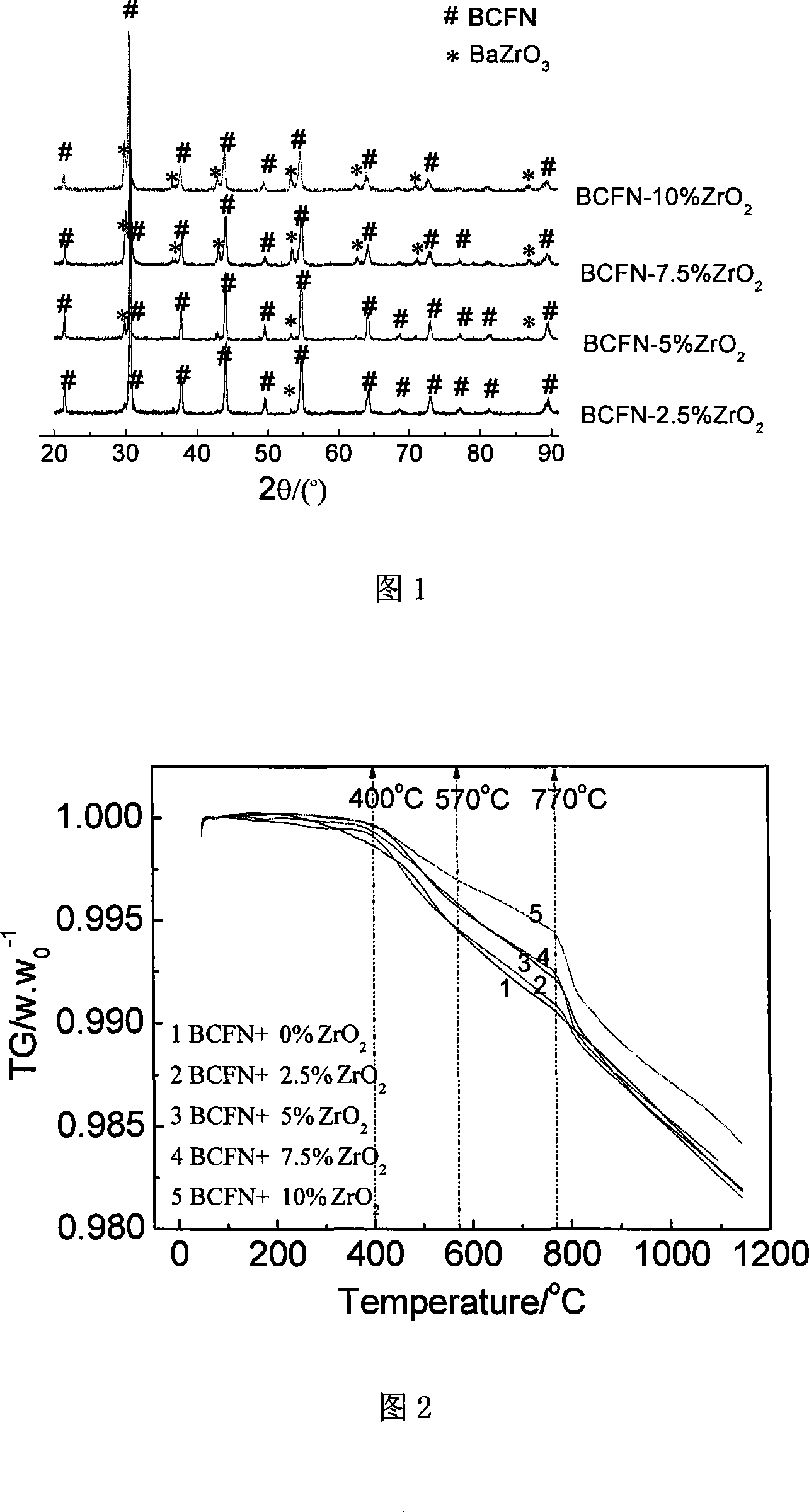
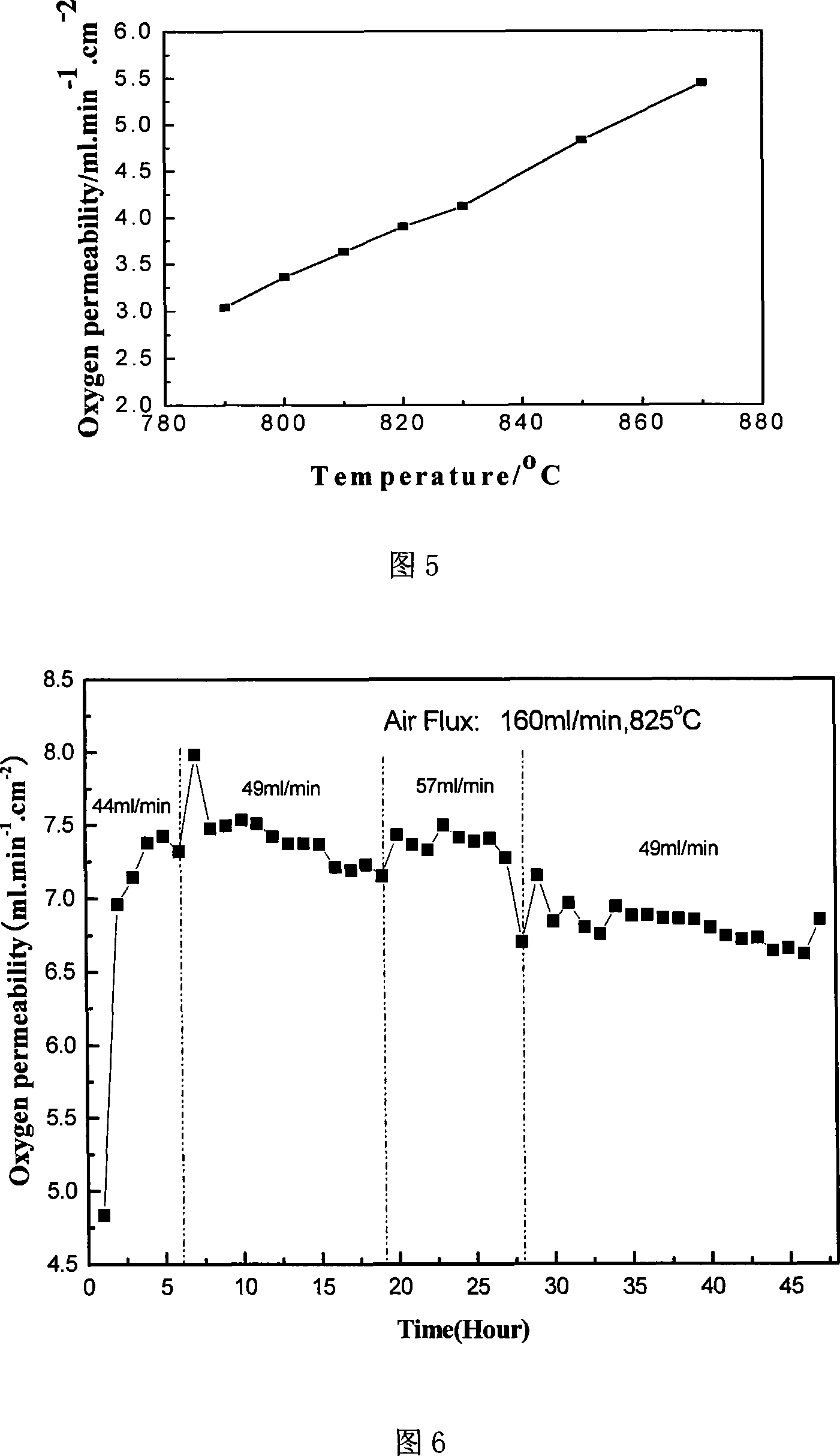
[0035] Embodiment 2: the different ZrO obtained in embodiment 1 2 The doped BCFN material was subjected to a TG experiment under an argon atmosphere, as shown in Figure 2. It can be seen from the figure that there are obvious differences in the weight loss of different samples under this atmosphere. The weight loss of samples with doping amount of 0wt% and 2.5wt% is similar, reaching 0.929% at 770℃. The weight loss of 5wt% and 7.5wt% is similar, reaching 0.776% at 770°C, and the weight loss of the 10wt% sample is the smallest, reaching 0.561% at 770°C. The change in weight loss of doped samples between 400°C and 770°C is different on both sides of 570°C; the weight loss rate is faster between 400°C and 570°C, and the weight loss rate is relatively slow between 570°C and 770°C. The sudden change in the weight loss curve of the ℃ material may be caused by a large amount of desorption of internal lattice oxygen resulting in a change in the material structure, corresponding to th...
Embodiment 3
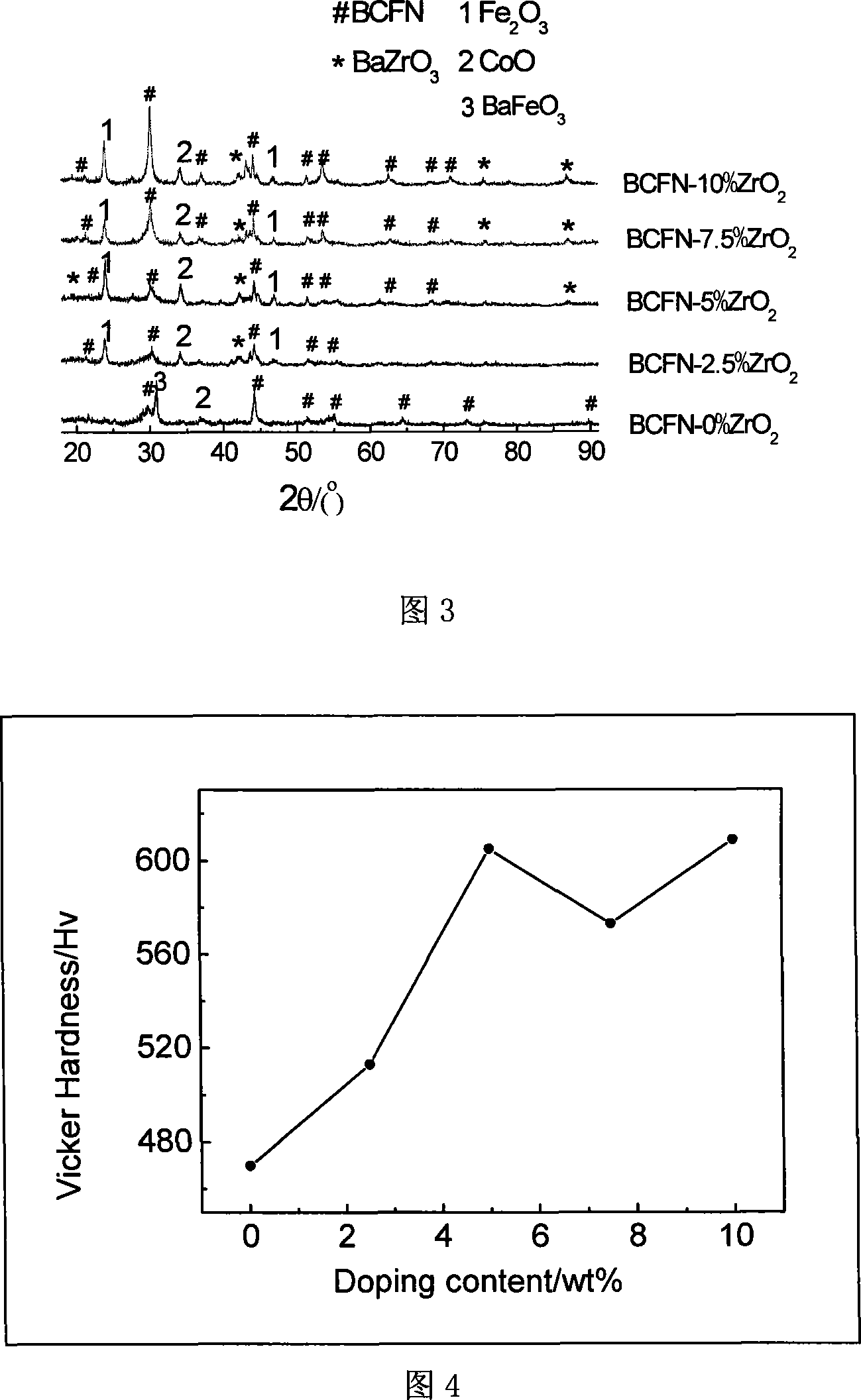
[0036] Embodiment 3: the different ZrO obtained in embodiment 1 2 After the doped sample was treated with 40% hydrogen, the phase structure changed obviously. It can be seen from the peak intensity variation of the strongest peak of the BCFN phase in different doped samples in Figure 3 that the doped samples of 7.5wt% and 10wt% are more stable under hydrogen than other samples. Although the pure BCFN sample retains part of the perovskite phase structure, the decomposition is serious. Except for the residual perovskite structure diffraction peak, only the diffraction peak of BaFeO3 is detected, because other impurity phases are too many and messy, so it is impossible to make fine According to the analysis, it is estimated that the impurity phase is mainly composed of CoO, Co, FeO and Fe2o3. For samples with different doping amounts, the diffraction peaks of the perovskite structure gradually become more obvious as the ZrO2 doping amount increases, indicating that the structura...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com