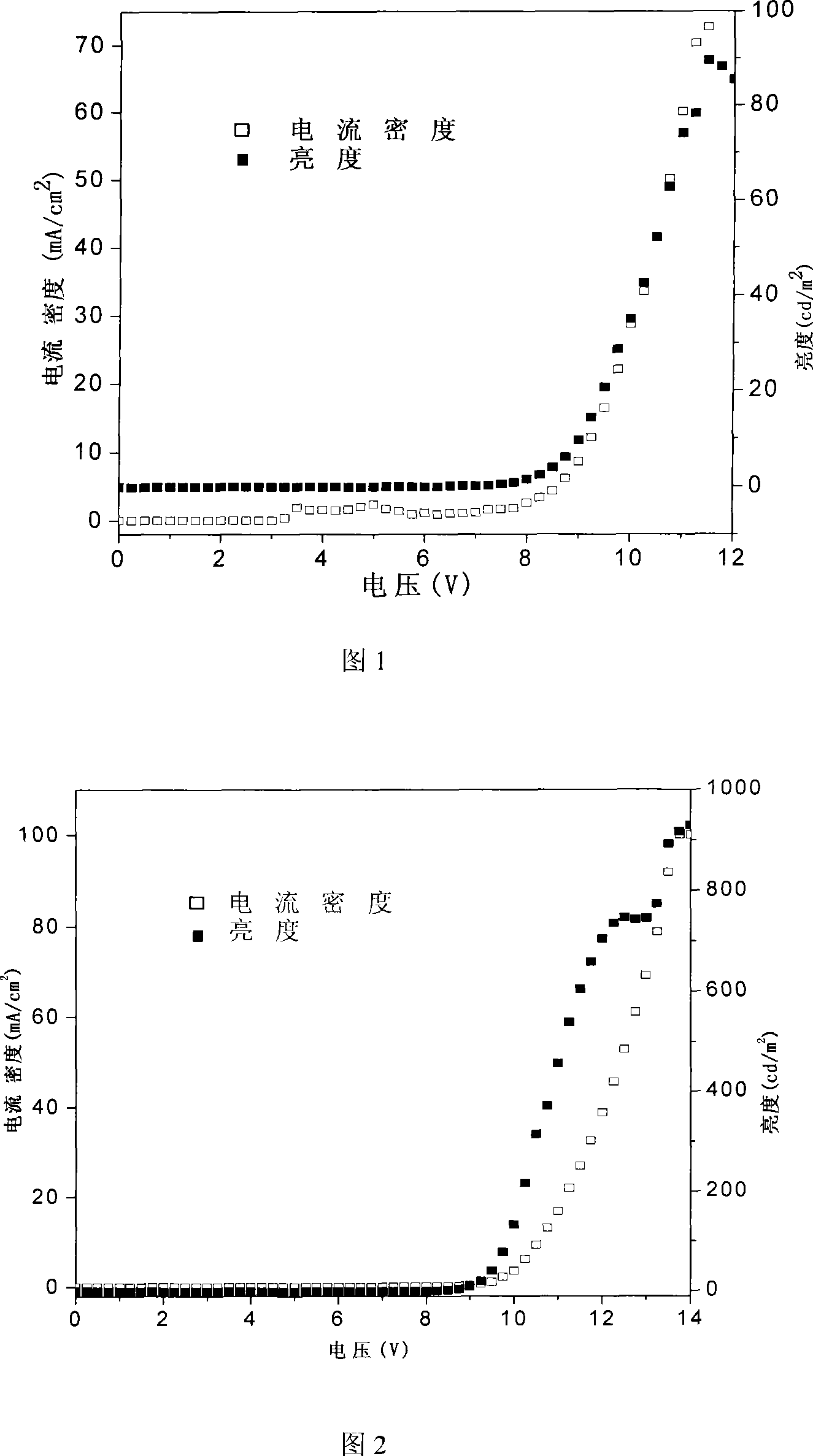
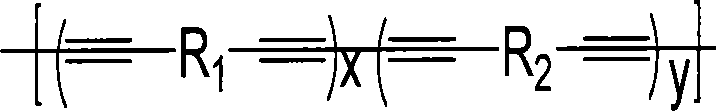Aryl containing diacetylene conjugated polymer and synthesis thereof
An aryl diacetylene-containing and conjugated polymer technology is applied in the field of aryl diacetylene-containing macromolecular polymers and their synthesis, which can solve the problems of low molecular weight and the like, and achieve high yield and molecular weight and good thermal stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] Synthesis of N-octylcarbazole:
[0035] Carbazole (6g, 35.9mmol), tetrabutylammonium bromide (1g, 3.1mmol), toluene (80ml), KOH solution (50%, 100ml), bromooctane (8.116ml) were added to 250ml (19#) In a two-necked round bottom flask, heat to reflux, stop the reaction after 30h. Extract with toluene, wash with water until neutral, add anhydrous Na 2 SO 4 After drying, filtering and rotary evaporation, 9.779 g of white viscous liquid was obtained, with a yield of 97.0%.
[0036] Synthesis of 3,6-diiodo-N-octylcarbazole:
[0037] N-octylcarbazole (3.767g, 13.5mmol), KI (4.001g, 24.1mmol), KIO 3 (4.836g, 22.6mmol), HOAc (35ml) was added to a 250ml (24#) three-neck round bottom flask, the oil bath was heated to 90°C in advance, and then the flask was immersed in the oil bath, and the temperature was rapidly raised to 120°C, the acetic acid Reflux, stop the reaction after 1h. Extracted with chloroform, followed by Na 2 S 2 o 3 The solution was washed wit...
Embodiment 2
[0046]
[0047] Synthesis of 2,7-dibromo-9,9-ditrianilinofluorene:
[0048] Add dibromofluorenone (2.46g, 7.38mmol) and triphenylamine (25g, 102mmol) into a 100ml three-necked round-bottomed flask, add 0.47ml of methanesulfonic acid with a syringe, heat to 150°C in a sand bath, and react for 7 hours. The reaction was stopped, extracted with dichloromethane, and the solvent was distilled off under reduced pressure, followed by column chromatography purification (developing solvent: petroleum ether) to obtain 2.8 g of a white powdery solid with a yield of 47%.
[0049] Synthesis of 2,7-bis(3,3-dimethyl-3-hydroxy-propynyl)-9,9-ditriphenylaminofluorene:
[0050] 2,7-dibromo-9,9-bistriphenylaminofluorene (0.86g, 1mmol), CuI trace, Pd(Ph 3 P) 2 Cl 2 (15mg, 0.02mmol) was sequentially added to a 100ml (19#) single-necked round bottom flask, and under nitrogen protection, Et 3 N (15ml) and toluene (10ml) were added, and 3,3'-dimethyl-3-hydroxypropyne (0.4ml) was added. The reac...
Embodiment 3
[0057]
[0058] Bis-(4-iodophenyl)-4-octyloxyaniline:
[0059] Diphenyl-4-octyloxyaniline (1.4g, 3.75mmol), KI (0.834g, 5.03mmol), KIO 3 (0.803g, 3.75mmol), HOAc (25ml) was added to a 250ml (24#) three-necked round-bottomed flask, the oil bath was heated to 90°C in advance, and then the flask was immersed in the oil bath, and the temperature was rapidly raised to 120°C. Reflux, stop the reaction after 1h. Extracted with chloroform, followed by Na 2 S 2 o 3 The solution was washed with chloroform to remove excess iodine, and anhydrous MgSO was added 4 Dry, filter, and purify by column chromatography (developing solvent: petroleum ether) to obtain 1.377 g of white crystals with a yield of 63.4%.
[0060] Di-[4-(3,3-dimethyl-3-hydroxy-propynyl)]phenyl-4-octyloxyaniline:
[0061] Bis-(4-iodophenyl)-4-octyloxyaniline (1.37g, 2.19mmol), CuI trace, Pd(Ph 3 P) 2 Cl 2 (16mg) was added to a 250ml single-necked round bottom flask in sequence, and under nitrogen protection, Et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com