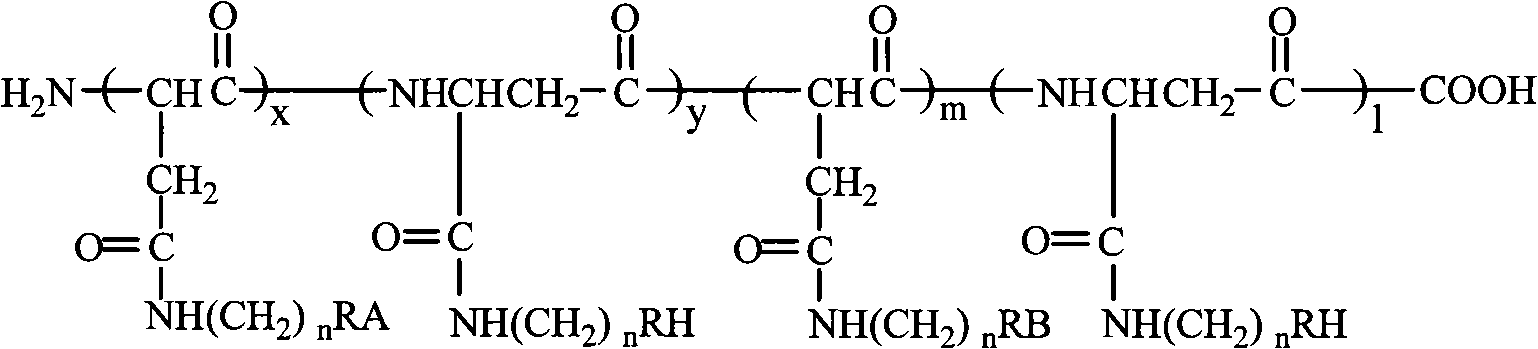
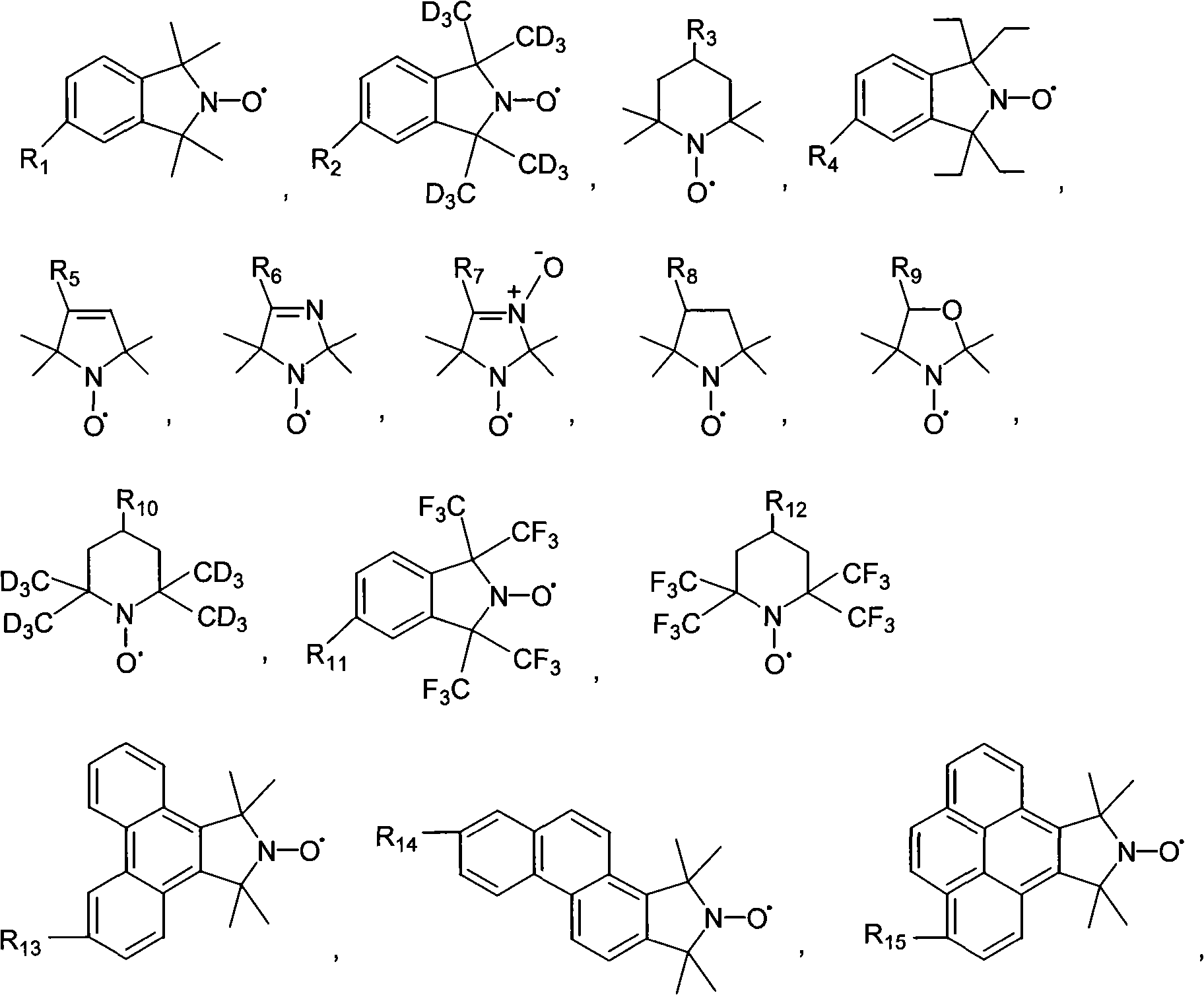
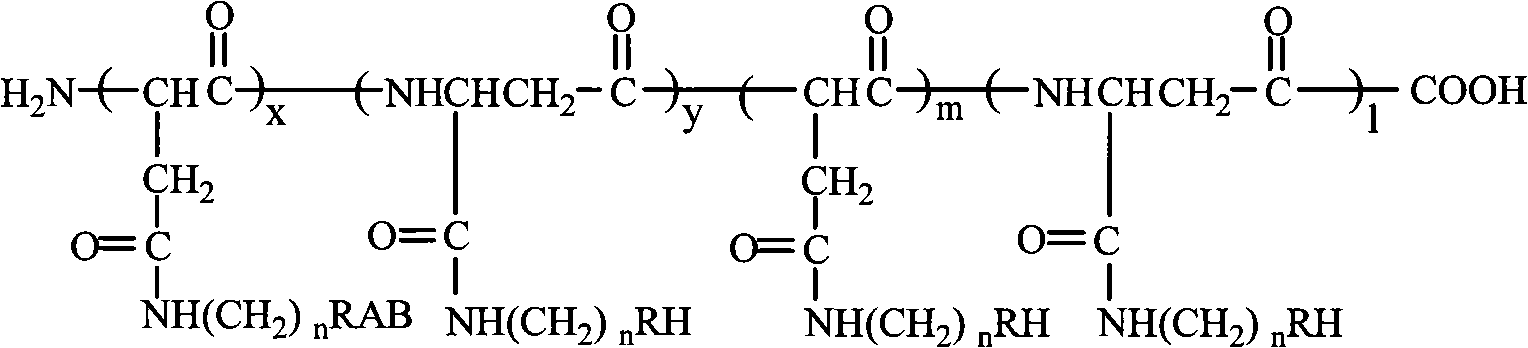Tumor targeting poly-asparagine free radical compounds and synthesizing method and use
A polyasparagine, tumor-targeting technology, used in the fields of chemistry and medicine, can solve the problems of short retention time, poor imaging effect, and no tissue or organ selectivity or targeting.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Tumor-targeting Polyasparagine Free Radical Compounds Containing Sulfadiazine
[0074] a) Reaction of sulfadiazine with bromoacetyl chloride
[0075] Dissolve 2.5g of sulfonamide (SN) in 40mL of N,N-dimethylformamide (DMF), add 2mL of triethylamine and stir, cool in an ice-water bath, slowly add 1.3g of bromoacetyl chloride dropwise under stirring, 0-5°C React for 2 hours, stir at room temperature for 4 hours, pass dry air to drive away the residual reaction gas, and store the reaction solution at 0-5°C for later use;
[0076] b) 5-Carboxylic acid-1,1,3,3-tetramethylisozaindene nitroxide free radical active ester
[0077]4.7g of 5-carboxylic acid-1,1,3,3-tetramethylisozaindene nitric oxide radical (20mmol) was dissolved in 30mL DMF and 2mL triethylamine mixed solvent, cooled to 0°C, and 4.1g N, N'-dicyclohexylcarbodiimide (20mmol), stirred at 0°C for 30 minutes, then added 2.3g of N-hydroxysuccinimide (20mmol), stirred at 0°C for 1 hour, and stirred at room temperatur...
Embodiment 2
[0081] Tumor-targeting polyasparagine free radical compounds containing sulfadiazine.
[0082] a) Preparation of 4-benzyloxycarbonyl amido-N-isethsulfadiazine and reaction with phosgene:
[0083] Dissolve 2.94g of 4-amino-N-isethsulfadiazine in 10mL of N,N-dimethylformamide, add 5mL of pyridine, cool in an ice-water bath at 0-5°C, add dropwise 5mL containing 1.7g of benzyloxy The benzyl alcohol solution of carbonyl chloride was reacted at 0-5°C for 4 hours and at room temperature for 6 hours; an appropriate amount of ether was added to precipitate, filtered, and vacuum-dried. The resulting white solid was dissolved in 50 mL N,N-dimethylformamide, and 2 mL Stir with ethylamine, cool in an ice-water bath, slowly add 4.5 mL of phosgene toluene solution dropwise under stirring, wherein the molar concentration of phosgene is 2 mol / mL, react at 0-5°C for 1 hour, stir at room temperature for 2 hours, and drive off with dry air Residual phosgene, the reaction solution was stored at 0...
Embodiment 3
[0090] Porphyrin-containing tumor-targeting polyasparagine free radical compounds.
[0091] a) 5-(4-aminophenyl)-10,15,20-three (4-sulfonic acid phenyl) porphyrin reacts with bromoacetyl chloride:
[0092] 4.68g 5-(4-aminophenyl)-10,15,20-tris(4-sulfonic acid phenyl)porphyrin was dissolved in 30mL N,N-dimethylformamide, added 2mL triethylamine and stirred, Cool in an ice-water bath, slowly add 0.69 bromoacetyl chloride dropwise under stirring, react at 0-5°C for 2 hours, stir at room temperature for 4 hours, drive the residual reaction gas with dry air, and store the reaction solution at 0-5°C for later use;
[0093] b) Preparation of 5-5-amino-1,1,3,3-tetradeuteromethylisozaindene nitroxide free radical active ester
[0094] 2.29g of 5-amino-1,1,3,3-tetradeuteromethylisozaindene nitric oxide radical was dissolved in 30mL of anhydrous diethyl ether and 10mL of dichloromethane mixed solvent, and 1g of N,N'-dichloromethane was added Dissolve cyclohexylcarbodiimide in 20 mL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com