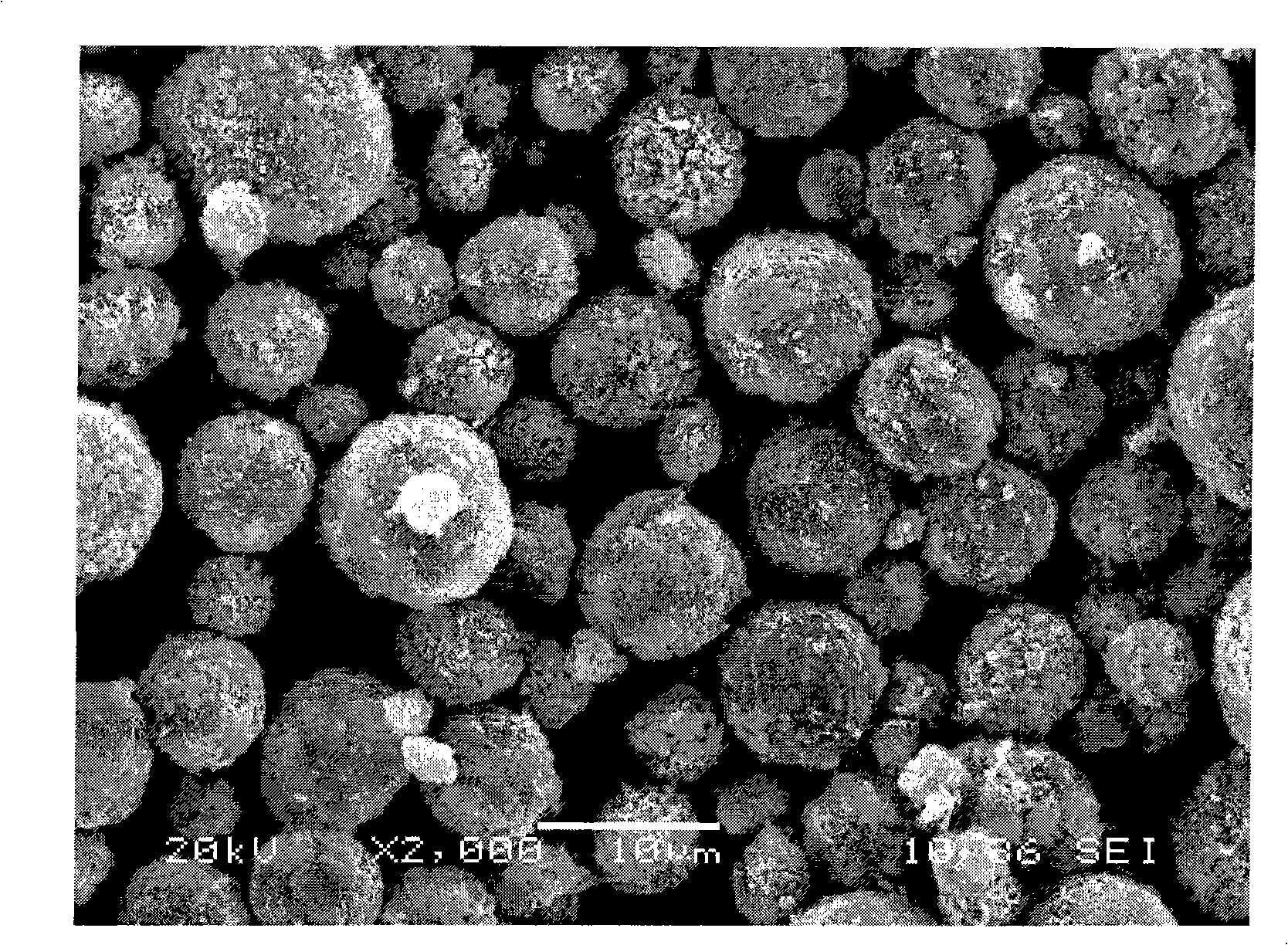
High-density spherical lithium nickel cobalt manganese oxygen and method for preparing the same
A lithium-nickel-cobalt-manganese-oxygen, high-density technology is applied in the field of lithium-nickel-cobalt-manganese-oxygen to achieve the effects of easy industrialization, easy cleaning, and increased volume specific capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Use deionized water to make cobalt sulfate into 1M, nickel sulfate 0.6M, manganese sulfate 1M, ammonium citrate into 2M mixed solution, use deionized water to make potassium hydroxide into 4M, ammonia water into 2M mixed solution, Turn on the stirring of the reaction vessel, put in 3 liters of deionized water, heat the bottom water to 40°C, add the nickel-cobalt-manganese solution into the reaction vessel at a flow rate of 5ml / min, and add the alkaline precipitant potassium hydroxide to 5-15ml The flow rate of / min is added to the reaction container, and the pH value is controlled by adjusting the flow rate of potassium hydroxide to 11.5±0.3. The precipitate suspension generated by the reaction overflows to another container for aging. After aging for 6 hours, the precipitate Wash until the pH of the washing water is less than 7.5, then filter, dry the filter cake at 90°C, weigh 90g of the dried product and 38.8g of lithium hydroxide, measure 50ml of deionized water, mix...
Embodiment 2
[0027] Use deionized water to make a mixed solution of cobalt chloride to 2M, nickel chloride to 0.5M, manganese chloride to 0.5M, and ammonium citrate to 1M, and deionized water to make potassium hydroxide to 4M and ammonia To form a 2M mixed solution, turn on the stirring of the reaction vessel, put in 3 liters of deionized water, and heat the bottom water to 50°C, the nickel-cobalt-manganese solution is added to the reaction vessel at a flow rate of 4ml / min, and the alkaline precipitant hydrogen Potassium oxide is added to the reaction vessel at a flow rate of 5-15ml / min. By adjusting the flow rate of potassium hydroxide, the pH value is controlled to 10.5±0.3, and the precipitate suspension generated by the reaction overflows into another container for aging. After 6 hours, the precipitate was washed until the pH of the washing water was less than 7.5, then filtered, the filter cake was dried at 120°C, 90g of the dried product and 39.8g of lithium carbonate were weighed, an...
Embodiment 3
[0029] Use deionized water to make a mixed solution of cobalt chloride to 1M, nickel chloride to 0.8M, manganese chloride to 0.8M, and ammonium oxalate to 0.5M, and deionized water to make sodium hydroxide to 7M and ammonia To form a 1M mixed solution, turn on the stirring of the reaction vessel, put in 3 liters of deionized water, and heat the bottom water to 40°C, the nickel-cobalt-manganese solution is added to the reaction vessel at a flow rate of 10ml / min, and the alkaline precipitant hydrogen Sodium oxide is added to the reaction vessel at a flow rate of 5-15ml / min. By adjusting the flow rate of sodium hydroxide, the pH value is controlled to be 10±0.3, and the precipitate suspension generated by the reaction overflows into another container for aging. After 6 hours, wash the precipitate until the pH of the washing water is less than 7.5, then filter, dry the filter cake at 95°C, weigh 105g of the dried product and 40g of lithium carbonate, measure 55ml of deionized water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com