Alpha-methylnaphthalene purifying technique
A technology of methylnaphthalene and a purification method, which is applied in the technical field of purifying α-methylnaphthalene, can solve problems such as the difficulty in achieving the purity of α-methylnaphthalene, complex components and difficult raw material purification, and achieve low price and high product quality. The effect of good quality and wide range of solvent sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
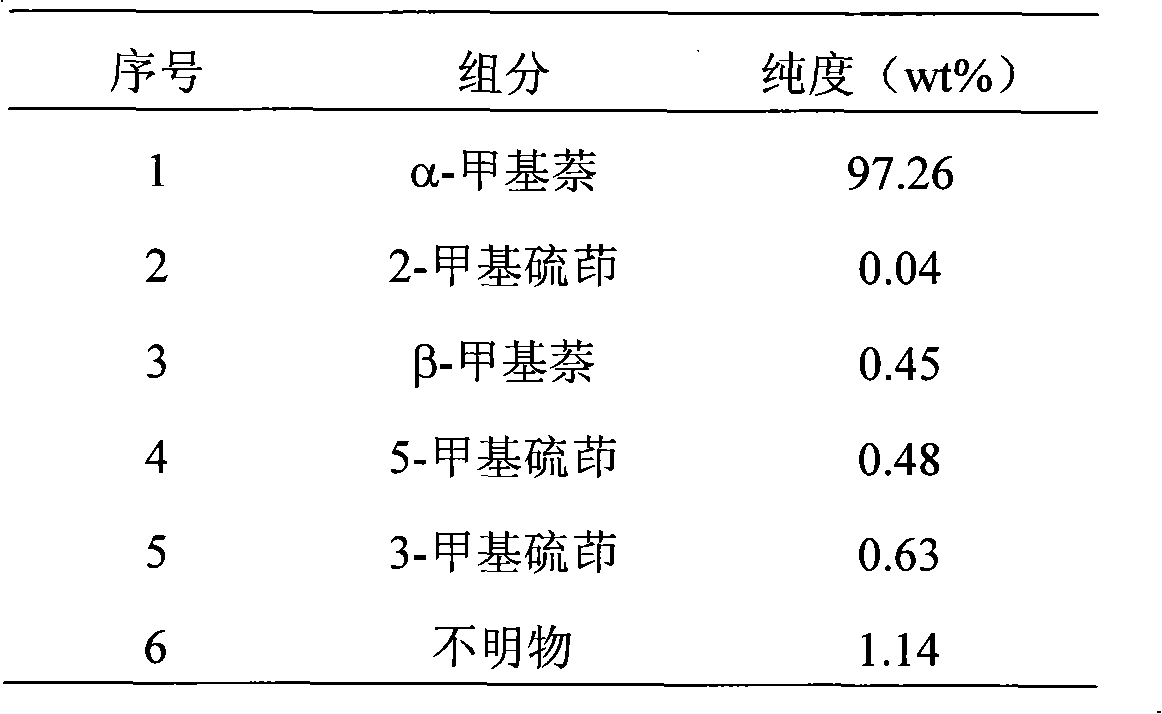
[0032] The α-methylnaphthalene raw material (composition sees Table 1) that 50g purity is 97.26% of indole joins in the 100ml Erlenmeyer flask, puts into magnetic stirrer, adds powdered anhydrous aluminum trichloride (raw material weight 1.14%), fully stirred, after reacting for 3.5 hours, the solid was filtered off, and the filtrate was taken for analysis. The results are shown in Table 2. In the filtrate, 50 g of dehydrated alcohol (solvent: filtrate ≈ 1: 1) was added to the filtrate. ), transferred to the separatory funnel after fully oscillating, left to stand for 25 minutes, and the upper layer liquid was taken for analysis, the results are shown in Table 3.
[0033] Quantitative analysis of the product was carried out on a Shimadzu GC-2010 gas chromatograph. The chromatographic column was a DB-WAX capillary column with a length of 30 m, an inner diameter of 0.25 mm, and a film thickness of 0.25 μm.
[0034] Table 1 removes the α-methylnaphthalene raw material composition...
Embodiment 2
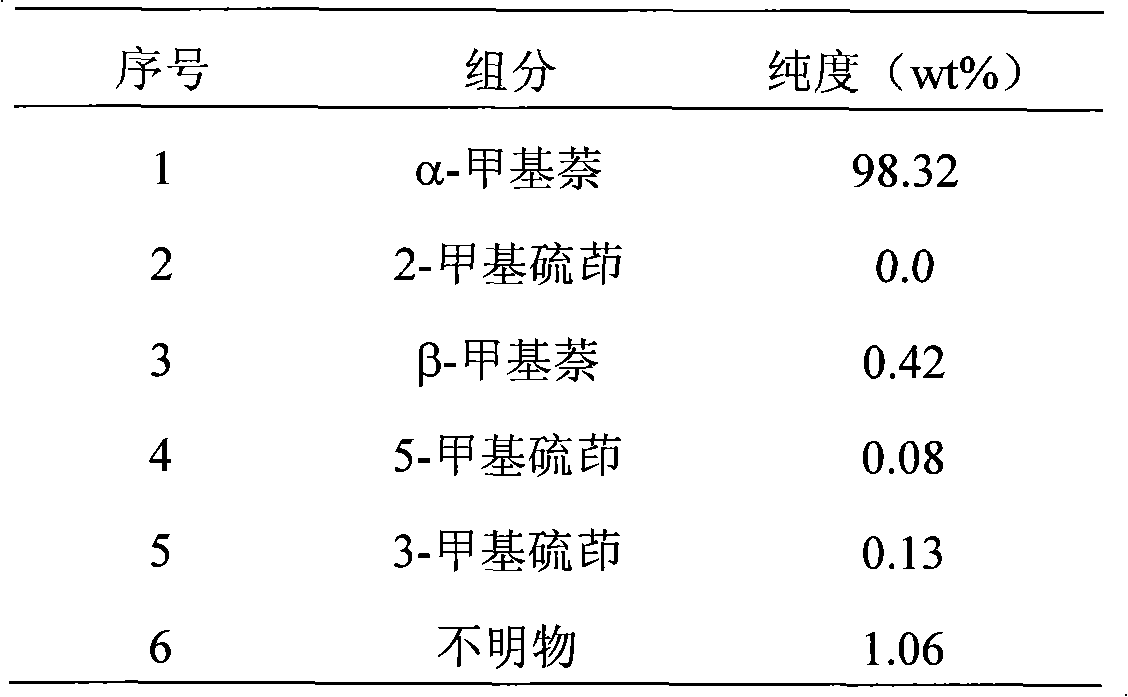
[0041] The α-methylnaphthalene raw material (composition is shown in Table 1) that 50g purity is 97.26% to remove indole joins in the 100ml Erlenmeyer flask, puts into magnetic stirrer, adds powdery anhydrous aluminum trichloride (raw material 1.45% of the weight), fully stirred, after reacting for 5 hours, filter out the solid in the α-methylnaphthalene, get the filtrate and analyze, the results are shown in Table 4, add heated 42 ℃ dehydrated alcohol 90g in the filtrate (solvent: filtrate ≈ 1.8: 1), after fully oscillating, transfer it to a separatory funnel, let it stand for 30 minutes, get the upper layer liquid for analysis, the results are shown in Table 5, after the upper layer liquid is distilled off to remove the solvent, α-formazan The base naphthalene product weighs 44.8g, and the analytical results of the product are shown in Table 6.
[0042] Quantitative analysis of the product was carried out on a Shimadzu GC-2010 gas chromatograph. The chromatographic column wa...
Embodiment 3
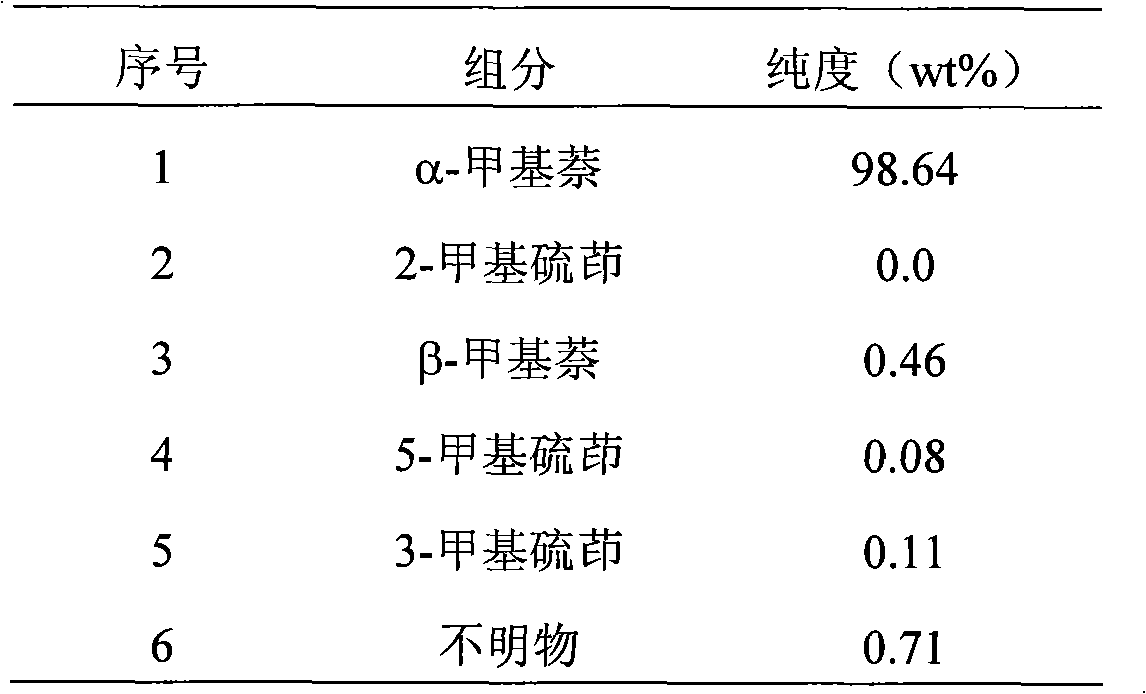
[0050] The α-methylnaphthalene raw material (see Table 1 for composition) that 40g purity is removed indole joins in the 100ml Erlenmeyer flask, puts into magnetic stirrer, adds powdered anhydrous zinc chloride (raw material weight 1.73%), fully stirred, after reacting for 6.5 hours, filter out the solid in the α-methylnaphthalene, get the filtrate and analyze, the results are shown in Table 7, add heated methanol 80g at 39°C in the filtrate (solvent: Filtrate ≈ 2: 1), after fully oscillating, transfer in the separatory funnel, leave standstill for 40 minutes, get the upper layer liquid and analyze, its result is shown in Table 8, after the upper layer liquid is distilled off solvent, obtains α-methyl naphthalene product , weighing 36.2g, the analytical results of the product are shown in Table 9.
[0051] Quantitative analysis of the product was carried out on a Shimadzu GC-2010 gas chromatograph. The chromatographic column was a DB-WAX capillary column with a length of 30 m, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com