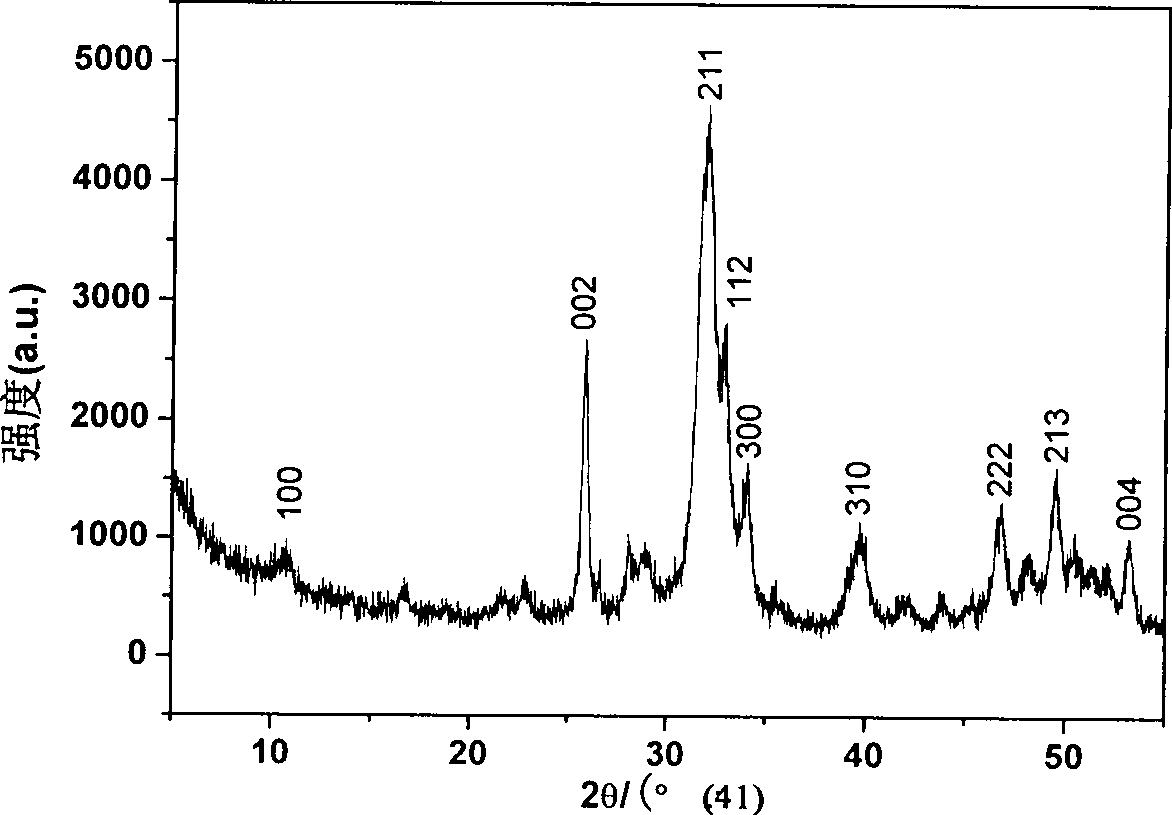
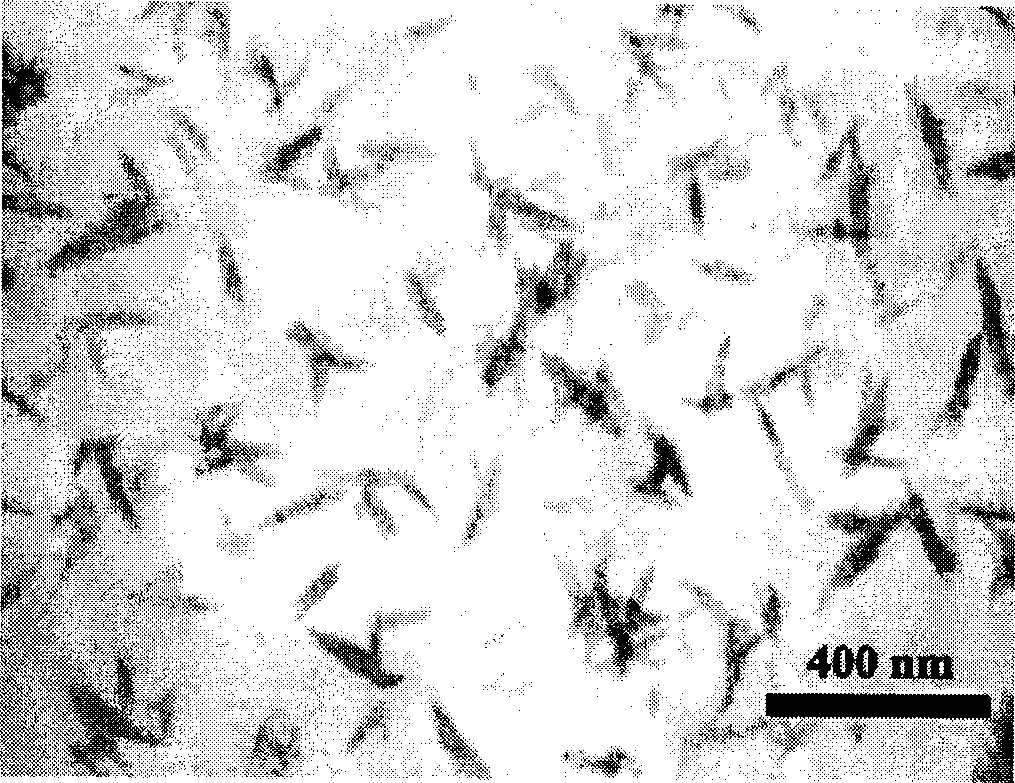
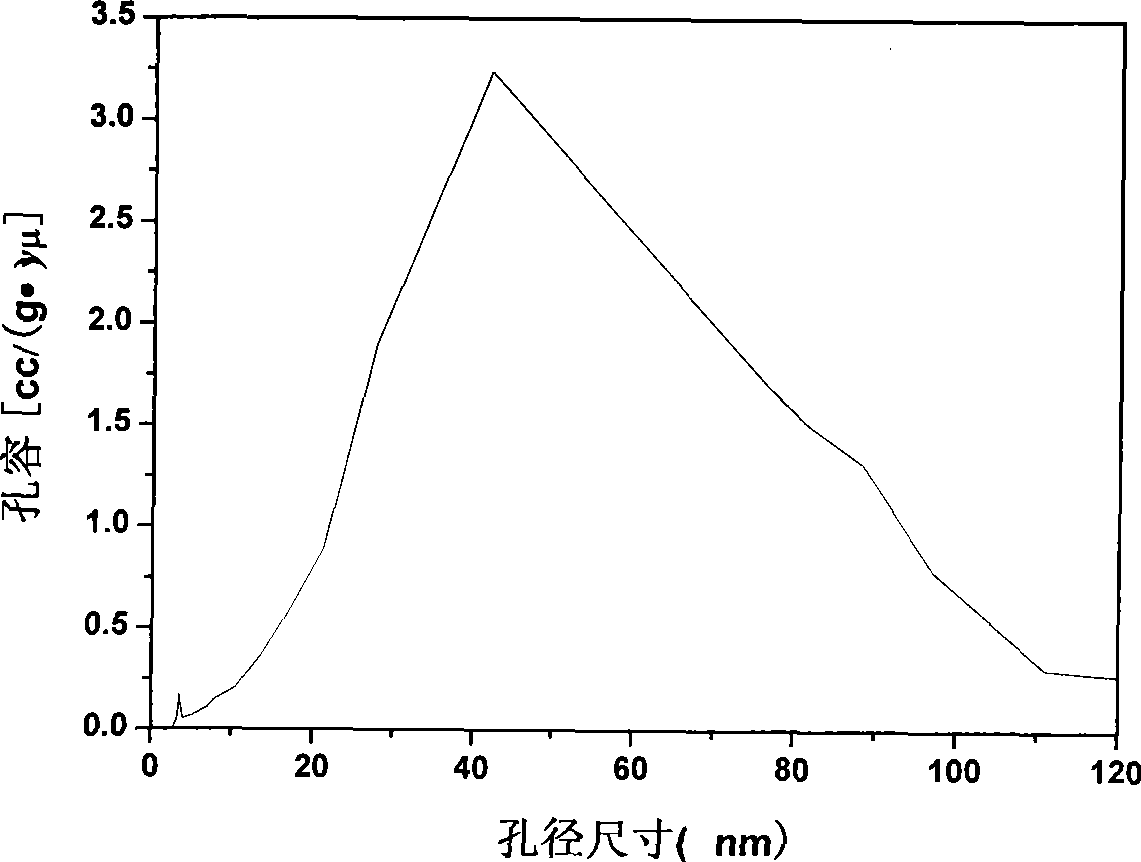
Controllable slow-releasing nano hydroxyapatite with efficient absorption for antalzyme and preparation method thereof
A nano-hydroxyapatite and hydroxyapatite technology, which is applied in the direction of medical preparations containing active ingredients, inorganic non-active ingredients, phosphorus compounds, etc., can solve the problem of insufficient degradation and absorption capacity, high water solubility of lysozyme, Problems such as application limitations, to achieve the effect of controllable release time, simple and easy operation, and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1. The adsorption amount of lysozyme is 4.77%~19.06%, the sustained release time is adjustable within 90~960min, and the method with the highest sustained release efficiency is 95%
[0025] Prepare respectively 50 mL of calcium hydroxide (Shanghai Chemical Reagent Company, China Pharmaceutical Group) suspension with a concentration of 0.5 mol / L and 50 mL of ammonium dihydrogen phosphate (Shanghai Chemical Reagent Company, China Pharmaceutical Group) aqueous solution with a concentration of 0.3 mol / L. Ammonium dihydrogen aqueous solution is added dropwise to the constantly stirring calcium hydroxide suspension at a certain rate (0.02ml / S), and the stirring rate is 180rad / min. After the dropwise addition, 50ml of absolute ethanol is added dropwise at the same rate into the reaction solution, and the reaction was carried out at room temperature. After 30 minutes of reaction, the resulting emulsion was left to age at room temperature for 24 hours, and then the pre...
Embodiment 2
[0028] Embodiment 2. The adsorption amount of lysozyme is 3.58%~17.36%, the sustained release time is adjustable within 90~360min, and the method with the highest sustained release efficiency is 87%
[0029]Preparation concentration is 50mL of calcium nitrate (Shanghai Chemical Reagent Company of China National Pharmaceutical Group) aqueous solution of 0.5mol / L, and adjusts pH=10.71 with ammonia water; 50mL, add ammonia water to adjust pH=10.36. Under the condition of a water bath at 40°C, add the diammonium hydrogen phosphate aqueous solution dropwise at a certain rate (0.02ml / S) into the calcium nitrate water suspension which is constantly stirring, and the stirring rate is 180 rpm. Adjust the pH of the reaction product with ammonia water to 10, continue to stir and react for 30 minutes, and leave the resulting emulsion to age in a water bath at a constant temperature of 40°C for 24 hours, then repeatedly wash the precipitated product with deionized water until it is neutral...
Embodiment 3
[0031] Take the lysozyme suspension adsorbed at 1.0mg / ml, accurately weigh 0.6g of the powder material loaded with lysozyme, and dissolve it in 400ml of acetate buffer solution with a concentration of 0.2mol / L and a pH distribution of 4.0 to 7.3 , stirred and desorbed at a constant temperature of 37°C, until the Ca in the solution 2+ until the ion concentration no longer increases. The release time is adjustable from 90 to 360 minutes, and the release rate is maintained above 60%, up to 87%. Among them, the sustained-release time and the highest release rate of the acetate buffer solution with pH=5.4 were the longest. Embodiment 3. The method that the adsorption amount of lysozyme is 2.14%-15.13%, the slow-release time is adjustable within 60-240min, and the maximum slow-release rate is 75%.
[0032] Preparation concentration is 50mL of calcium nitrate (Shanghai Chemical Reagent Company of China National Pharmaceutical Group) aqueous solution of 0.5mol / L, and adjusts pH=10.7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com