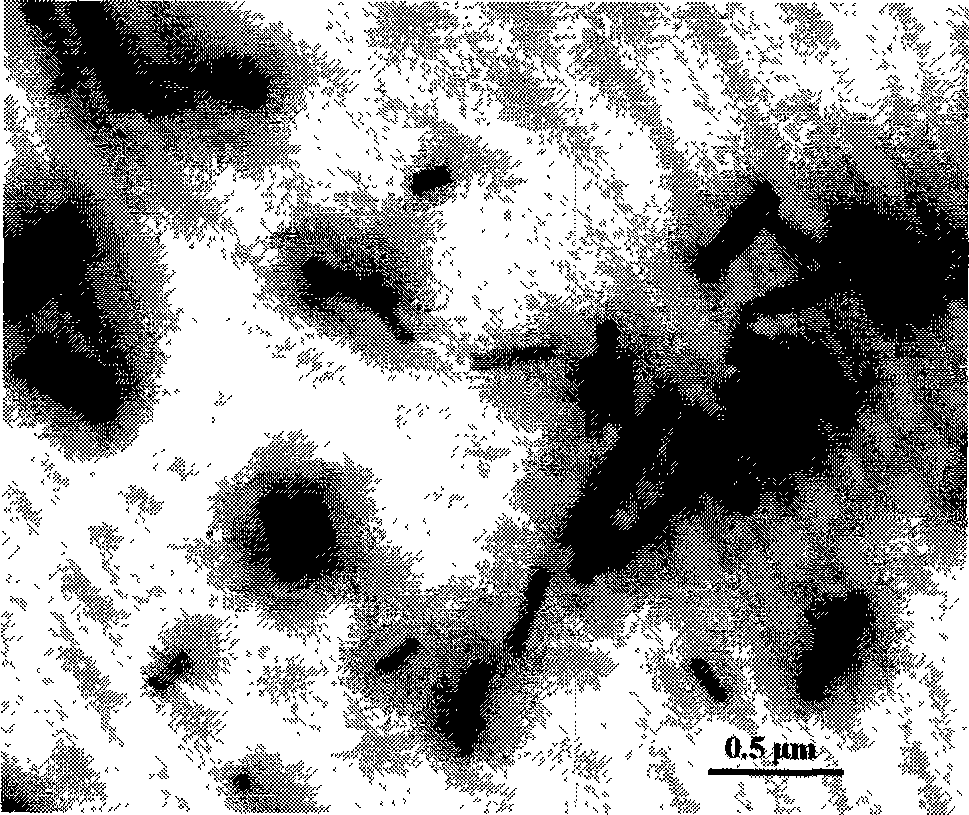
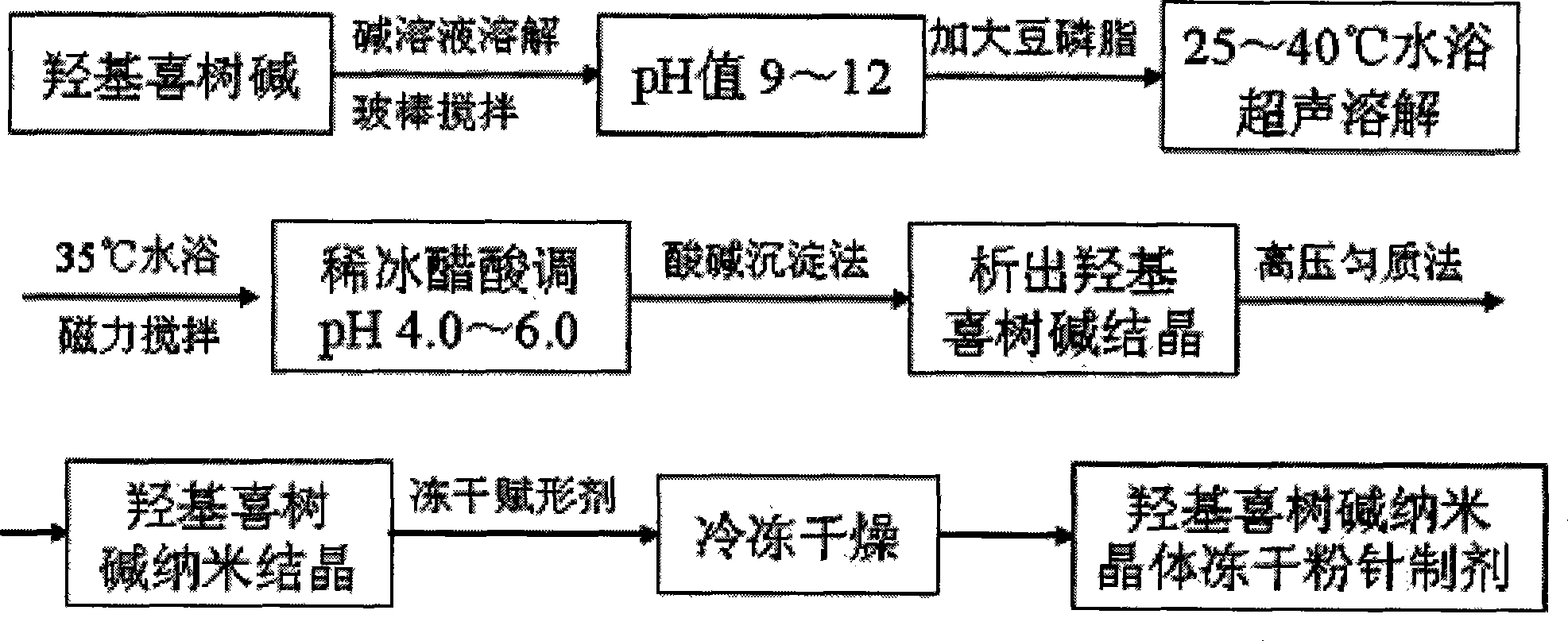
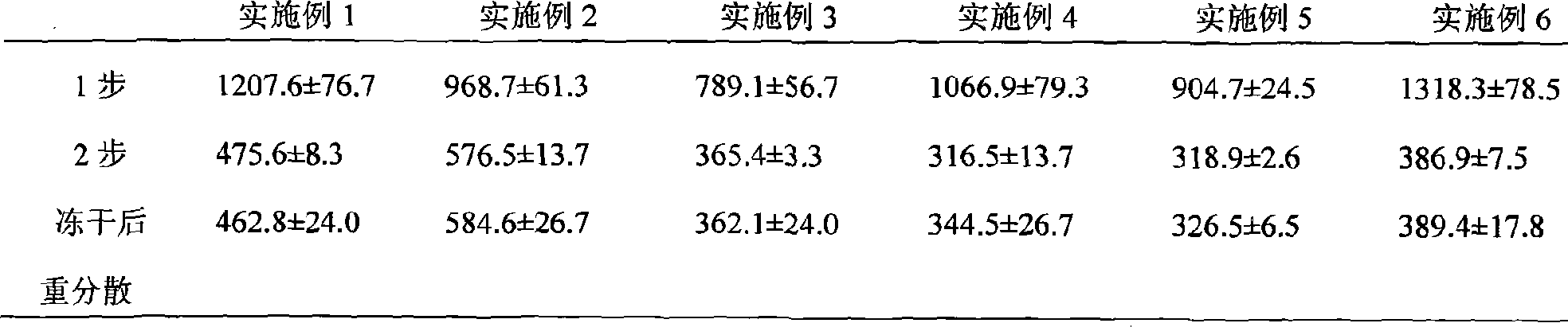
Hydroxycamptothecin nano crystal lyophilized powder for injection preparation and preparation method thereof
A technology of hydroxycamptothecin and nanocrystals, which is applied in the direction of freeze-drying transportation, powder transportation, pharmaceutical formulations, etc., can solve the problems of limiting clinical application, decreasing pharmacological activity, and prolonging the course of treatment, so as to improve the therapeutic effect, the preparation method is simple, The effect of prolonging the survival time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) The main raw materials for preparing hydroxycamptothecin nanocrystal freeze-dried powder preparation:
[0031] Hydroxycamptothecin 1 part by weight,
[0032] 2 parts by weight of soybean lecithin,
[0033] Surfactant poloxamer 188 1 part by weight,
[0034] 0.5 parts by weight of mannitol.
[0035] (2) The method for preparing hydroxycamptothecin nanocrystal freeze-dried powder preparation:
[0036] Dissolve 1 part by weight of hydroxycamptothecin in a 100ml container to prepare 90ml of an aqueous solution, add sodium hydroxide to adjust the pH to 9, stir and dissolve with a glass rod, then add 2 parts by weight of soybean lecithin, and ultrasonicate in a water bath at 25°C for 10 Minutes to dissolve; 35 ℃ water bath magnetic stirring, add 1 weight part of surfactant poloxamer 188 to dissolve; use glacial acetic acid to adjust the pH value to 4.0; 100ml, divided into vials, 2ml per bottle, freeze-dried to obtain the finished product.
Embodiment 2
[0038] (1) The main raw materials for preparing hydroxycamptothecin nanocrystal freeze-dried powder preparation:
[0039] Hydroxycamptothecin 2 parts by weight,
[0040] 4 parts by weight of egg yolk lecithin,
[0041] Surfactant macrogol stearate-155 parts by weight,
[0042] 1 part by weight of mannitol.
[0043] (2) The method for preparing hydroxycamptothecin nanocrystal freeze-dried powder preparation:
[0044] Dissolve 2 parts by weight of hydroxycamptothecin in a 100ml container to prepare 90ml of an aqueous solution, add sodium hydroxide to adjust the pH to 12, stir and dissolve with a glass rod, then add 4 parts by weight of soybean lecithin, and ultrasonicate in a water bath at 40°C for 1 Minutes to dissolve; 33 ℃ water bath magnetic stirring, adding 5 parts by weight of surfactant poloxamer 188 to dissolve; use glacial acetic acid to adjust the pH value to 6.0; 100ml, divided into vials, 2ml per bottle, freeze-dried to obtain the finished product.
Embodiment 3
[0046] (1) The main raw materials for preparing hydroxycamptothecin nanocrystal freeze-dried powder preparation:
[0047] 3 parts by weight of hydroxycamptothecin,
[0048] 6 parts by weight of soybean lecithin,
[0049] Polyethylene glycol stearate-15 6 parts by weight,
[0050] 5 parts by weight of glucose for injection.
[0051] (2) The method for preparing hydroxycamptothecin nanocrystal freeze-dried powder preparation:
[0052] Dissolve 3 parts by weight of hydroxycamptothecin in a 100ml container to prepare 90ml of an aqueous solution, add sodium hydroxide to adjust the pH value to 10, stir and dissolve with a glass rod, then add 6 parts by weight of soybean lecithin, and ultrasonicate in a water bath at 30°C for 5 Minutes to dissolve; 34 ℃ water bath magnetic stirring, add 6 weight parts of surfactant poloxamer 188 to dissolve; use glacial acetic acid to adjust the pH value to 5.0; 100ml, divided into vials, 2ml per bottle, freeze-dried to obtain the finished produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com