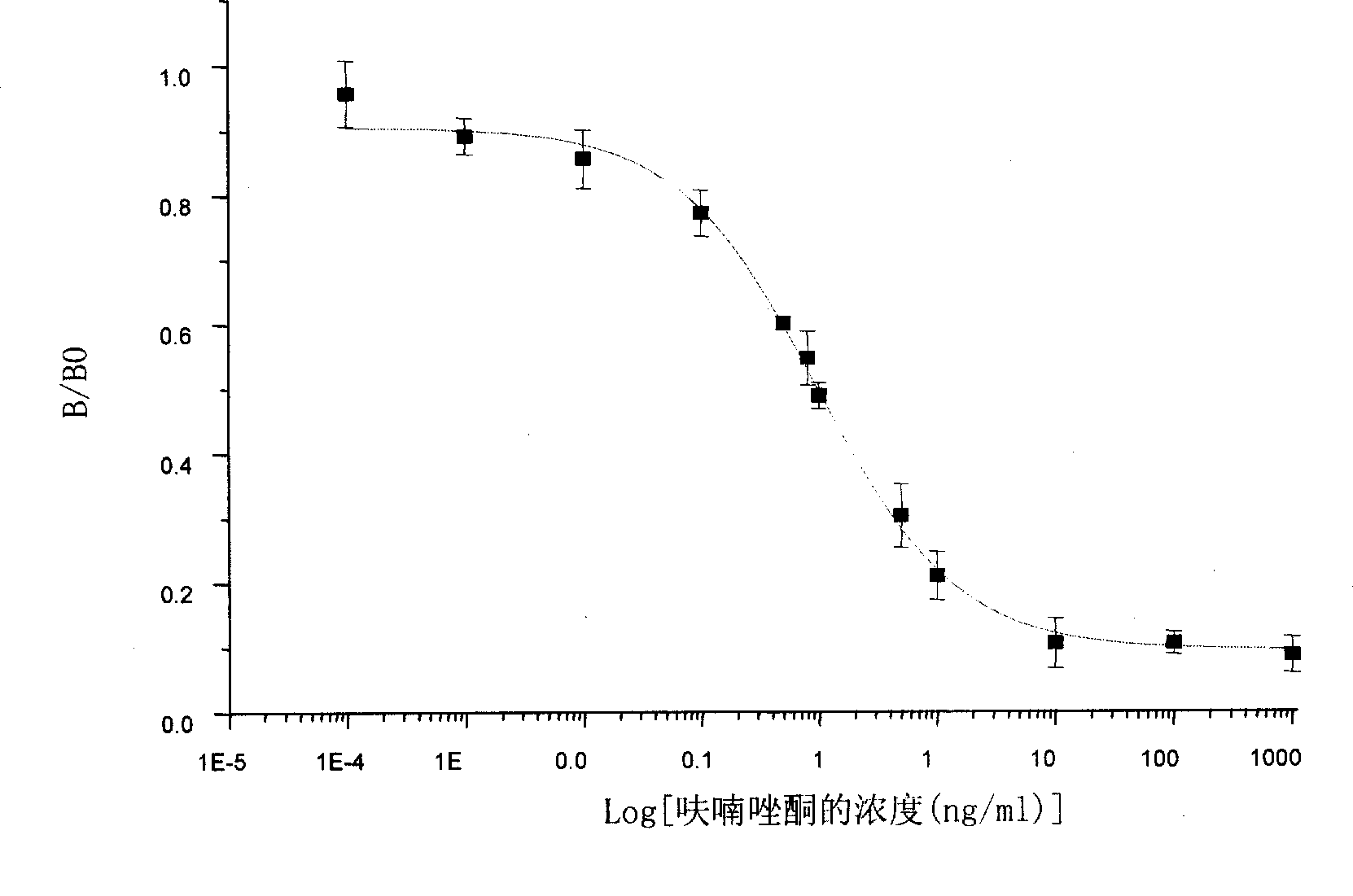
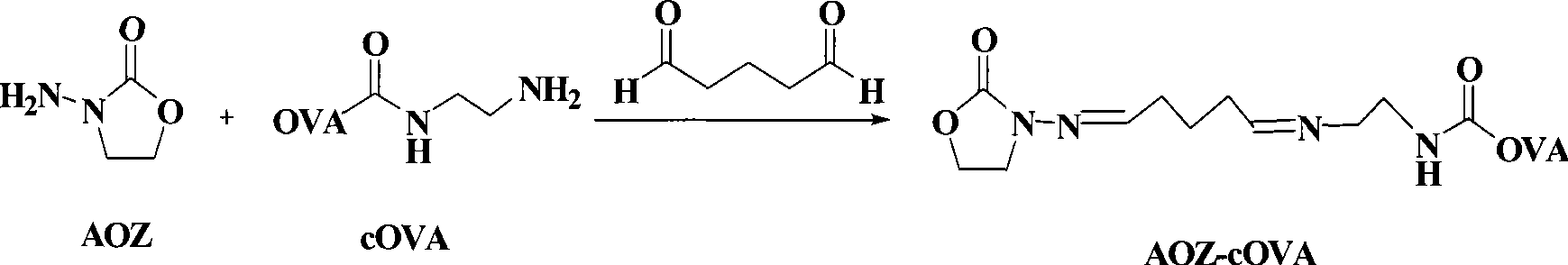
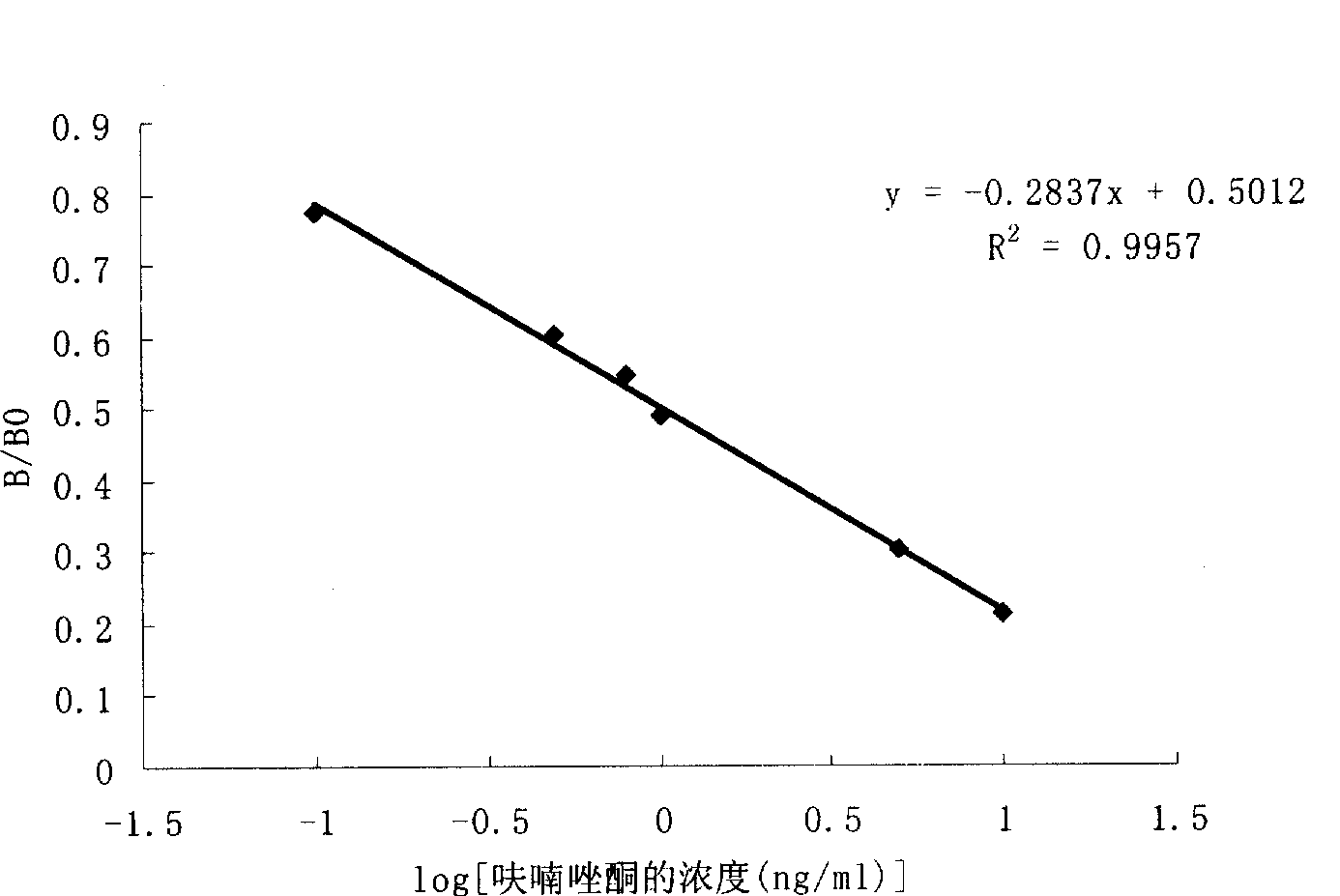Chemical luminescence ELISA detection reagent kit for furazolidone
A technology of chemiluminescence enzyme and furazolidone, which is applied in the fields of chemiluminescence/bioluminescence, analysis by causing chemical reactions of materials, and measuring devices, etc., which can solve the problems of cumbersome derivatization, time-consuming sample processing process, expensive and bulky instruments that are difficult to carry, etc. , to achieve the effect of simple sample pretreatment, low detection limit and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0028] Example 13- Derivatization of amino-2-oxazolone (AOZ), preparation of immunogen, coated antigen, and antibody
[0029] (1) Derivatization of AOZ
[0030] The preparation method of derivative CPAOZ of AOZ is as follows:
[0031] Dissolve 75mg (0.5mmol) of 3-carboxybenzaldehyde in 5mL of methanol to obtain liquid A. Dissolve 51 mg of AOZ (0.5 mmol) in 15 mL of methanol to obtain liquid B. Mix liquids A and B, stir, and reflux at 65°C. Track by thin chromatography, and the reaction is completed in about 9 hours. After rotary evaporation to dryness, about 20 mL of ethanol was added to wash and filter with suction. The derivative CPAOZ of AOZ is obtained.
[0032] (2) Preparation of immunogen
[0033] The mixed acid anhydride method prepares furazolidone and its metabolite (AOZ) immunogen CPAOZ-cBSA steps as follows:
[0034] Weigh 50 mg (0.21 mmol) of CPAOZ and dissolve in 10 mL of anhydrous DMF (anhydrous N-N dimethylformamide) and stir to dissolve, add 77 μL (0.32 m...
Embodiment 2
[0041] Embodiment 2 The establishment of chemiluminescent enzyme-linked immunosorbent assay (CL-ELISA)
[0042] (1) Optimal concentration of antibody and coated antigen (square matrix)
[0043]Use 100μL / well in the longitudinal direction of the microplate plate, and the concentration gradient is 80.0μg / mL, 40.0μg / mL, 20.0μg / mL, 10.0μg / mL, 5.0μg / mL, 2.5μg / mL, 1.25μg / mL, and 0.625 Coat with μg / mL coating antigen solution, place overnight at 4°C, wash the plate three times with 280 μL / well washing solution, then block with 250 μL / well blocking solution, place at room temperature for 2.5 hours, wash three times; add 100 μL / well horizontally, Dilute the antibody solution at 1:100, 1:200~1:51200, place at room temperature for 2 hours, wash three times; add 100 μL / well of 1:1000 horseradish peroxidase-labeled goat anti-rabbit antibody, place at room temperature Wash three times for 1 hour; add 100 μL / well of luminescence solution, and measure the luminescence value. Specificity det...
Embodiment 3
[0053] Embodiment 3, the assembly of the chemiluminescent ELISA kit for detecting furazolidone of the present invention
[0054] (1) The composition of the chemiluminescent ELISA kit for detecting furazolidone
[0055] a. A solid-phase carrier (milky white opaque polystyrene 96-well chemiluminescence microplate plate) coated with a coated antigen (a conjugate of AOZ and ovalbumin);
[0056] b. Furazolidone antibody working solution (volume ratio concentration is 1:4000);
[0057] c. 6 bottles of furazolidone standard solution, the concentrations are 0.1ng / mL, 0.5ng / mL, 0.8ng / mL, 1ng / mL, 5ng / mL and 10ng / mL;
[0058] d. Horseradish peroxidase-labeled goat anti-rabbit IgG antibody working solution (working concentration is 1:1000);
[0059] e Concentrated Phosphate Buffer Solution:: NaCl 80g, KH 2 PO 4 2.0g, Na 2 HPO 4 .12H 2 o 2 29.0g, KCl 2.0g dissolved in 1000mL distilled water
[0060] f Concentrated washing solution: Tween 20 (Tween20) with a volume ratio concentr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| coefficient of variation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com