Method for synthesizing 2-(3-cyano-4- isobutoxy phenyl)-4-methyl-carboxylate
A technology of isobutoxyphenyl thioformamide and isobutoxyphenyl, which is applied in the field of medicine, can solve the problems of unsuitability for industrial production, difficult availability of raw materials, and high production costs, and achieve low cost and easy availability of raw materials , the effect of less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
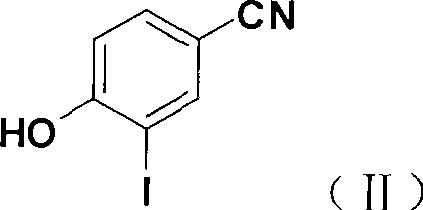
[0030] 1) 3-iodo-4-hydroxybenzonitrile
[0031] In a 250ml three-necked bottle, add 16.7g of potassium iodide, 21.3g of iodine, 86ml of water, and 14ml of concentrated ammonia water, stir mechanically at room temperature, add 10.0g of 4-hydroxybenzonitrile after dissolution, and react for 7 hours at room temperature. After the reaction was completed, the pH was adjusted to 1 with concentrated hydrochloric acid in an ice bath, and after standing for 1 hour, it was suction filtered, washed with water, and dried to obtain 18.8 g of a khaki solid, yield: 91.5%. ESI-MS m / z: 268[M+Na] + , 244[M-H] -
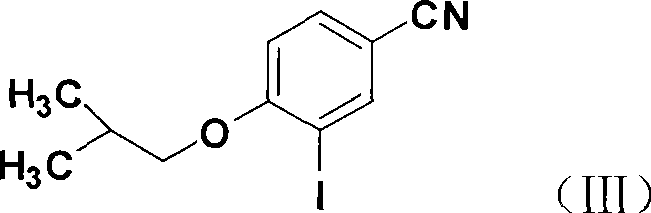
[0032] 2) 3-iodo-4-isobutoxybenzonitrile
[0033] In a 250ml round bottom flask, add 18.8g of 3-iodo-4-hydroxybenzonitrile, 15.9g of anhydrous potassium carbonate, 100ml of DMF, 3ml of PEG4003ml, stir at 60°C for 15min, then add 26.1g of bromoisobutane g, continue the reaction, raise the temperature to 80°C after 6h, and distill under reduced pressure for 1-2h (distill the excess i...
Embodiment 2
[0043] 1) 3-iodo-4-hydroxybenzonitrile
[0044] In a 250ml three-necked bottle, add 33.5g potassium iodide, 43.7g iodine, 172ml water, and 28ml concentrated ammonia water, stir mechanically at room temperature, add 20.0g 4-hydroxybenzonitrile after dissolution, and react for 7 hours at room temperature. After the reaction was completed, the pH was adjusted to 1 with concentrated hydrochloric acid in an ice bath, and after standing for 1 hour, it was suction filtered, washed with water, and dried to obtain 40.0 g of a khaki solid, yield: 97.1%.
[0045] 2) 3-iodo-4-isobutoxybenzonitrile
[0046] In a 250ml round bottom flask, add 40.0g of 3-iodo-4-hydroxybenzonitrile, 33.7g of anhydrous potassium carbonate, 200ml of DMF, 6ml of PEG, stir at 60°C for 15min, then add 55.5g of bromoisobutane g, to continue the reaction, raise the temperature to 80°C after 6h, and distill under reduced pressure for 1-2h (distill the excess isobutane bromide). Suction filtration, wash the filter c...
Embodiment 3
[0056] 1) 3-iodo-4-hydroxybenzonitrile
[0057] In a 1000ml three-necked bottle, add 100g of potassium iodide, 128g of iodine, 516ml of water, and 86ml of concentrated ammonia water, stir mechanically at room temperature, add 60.0g of 4-hydroxybenzonitrile after dissolution, and react for 7 hours at room temperature. After the reaction was completed, the pH was adjusted to 1 with concentrated hydrochloric acid in an ice bath, and after standing for 1 h, the mixture was suction filtered, washed with water, and dried to obtain 116.0 g of a khaki solid, yield: 94.3%.
[0058] 2) 3-iodo-4-isobutoxybenzonitrile
[0059]In a 1000ml round bottom flask, add 116.0g of 3-iodo-4-hydroxybenzonitrile, 98.0g of anhydrous potassium carbonate, 500ml of DMF, 10ml of PEG400, stir at 60°C for 15min, then add bromoisobutyl 160.1 g of alkanes continued to react, and after 6 hours, the temperature was raised to 80° C., and vacuum distillation was performed for 1 to 2 hours (excess bromoisobutane w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com