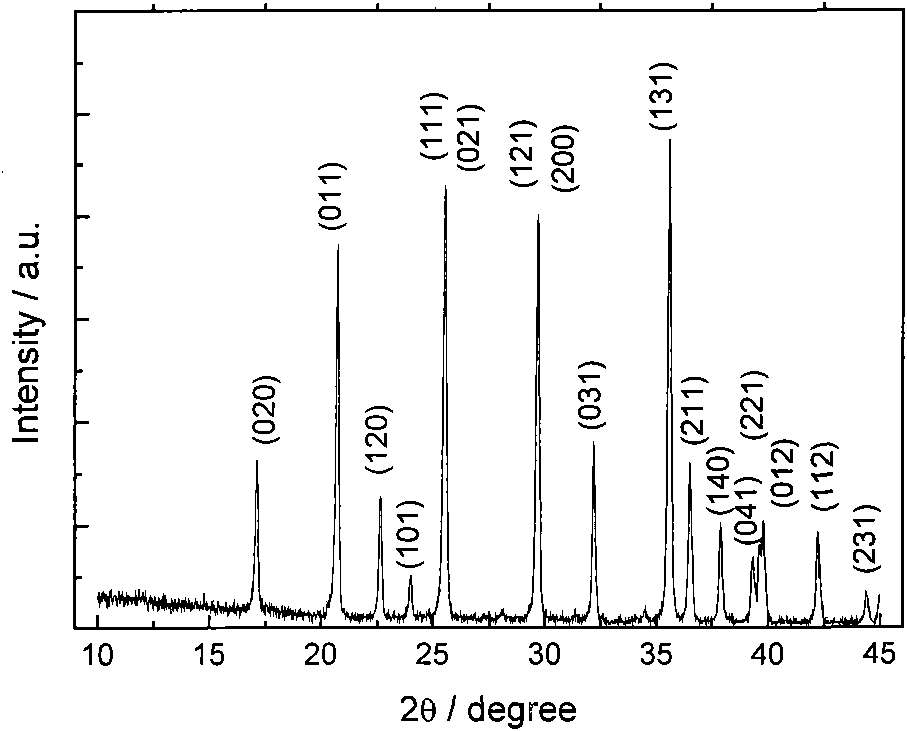
Method for preparing high-power lithium iron phosphate composite materials
A technology of lithium iron phosphate and composite materials, applied in phosphorus compounds, chemical instruments and methods, electrode manufacturing, etc., can solve problems such as restricting the application range of lithium iron phosphate batteries, decrease in battery energy density, heat generation, etc., and achieve large-scale industrialization. Production, excellent electrochemical performance, the effect of reducing the reaction barrier
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1. Under room temperature, 27g ferric nitrate Fe(NO3) 3.9H2O and 200ml concentrated nitric acid are uniformly mixed under the rotating speed of 600 revs / min, then add 0.5g carbon nanotube, ultrasonic dispersion, reflux 4.5 hours in the oil bath of 120 degree, will The temperature of the refluxed mixture is lowered to 20 degrees, and the pH value is adjusted to 10 with 3.0wt% ammonia solution and 50g of 3.2wt% lithium hydroxide solution, and stabilized for 5 minutes to obtain a suspension of iron hydroxide precursor embedded with carbon nanotubes liquid. Then slowly add 18ml of 1.5mol / L phosphoric acid and 20ml of 2.0mol / L diammonium hydrogen phosphate dropwise, stirring while adding dropwise; after the dropwise addition is completed, stop stirring. The reaction system was distilled under reduced pressure to obtain a lithium iron phosphate precursor embedded with carbon nanotubes. Grind the precursor powder and place it in a tube furnace, use 80% nitrogen and 2...
Embodiment 2
[0019] Example 2. At room temperature, 13.5g of ferric nitrate Fe(NO3)3.9H2O and 5.41g of ferric chloride FeCl3 were uniformly mixed with 200ml of concentrated nitric acid at a speed of 550 rpm, then 0.8g of carbon nanotubes were added, ultrasonically dispersed, and heated at 120 degrees Reflux in an oil bath for 4.5 hours, reduce the temperature of the refluxed mixture to 20 degrees, add 100 g of 2.48% lithium carbonate suspension, then adjust the pH value of the reaction solution to 9 with 4.0% wt of ammonia solution, and stabilize it for 5 minutes to obtain The ferric hydroxide precursor suspension embedded with carbon nanotubes; then slowly add 18ml of 1mol / L phosphoric acid and 49ml of 1mol / L diammonium hydrogen phosphate dropwise, stirring while adding; after the dropwise addition, stop stirring. The reaction system was distilled under reduced pressure to obtain a lithium iron phosphate precursor embedded with carbon nanotubes. Grind the precursor powder and place it in...
Embodiment 3
[0020] Example 3. Under room temperature, 36g ferric nitrate Fe(NO3) 3.9H2O and 200ml concentrated nitric acid are uniformly mixed under the rotating speed of 600 rpm, then add 0.8g carbon nanotube, ultrasonic dispersion, reflux 5 hours in the oil bath of 120 degree, will The temperature of the refluxed mixture was lowered to 25 degrees, and 100 g of a suspension solution containing 1.65 wt % lithium carbonate and 2.28 wt % lithium acetate was added, and then the pH value of the reaction solution was adjusted to 9 with 3.5 wt % ammonia water, and stabilized for 5 min to obtain Iron hydroxide precursor suspension embedded with carbon nanotubes; then slowly add 24ml of 1mol / L phosphoric acid and 65ml of 1mol / L diammonium hydrogen phosphate dropwise, and stir while adding; after the dropwise addition, stop stirring. The reaction system was distilled under reduced pressure to obtain a lithium iron phosphate precursor embedded with carbon nanotubes. Grind the precursor powder and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com