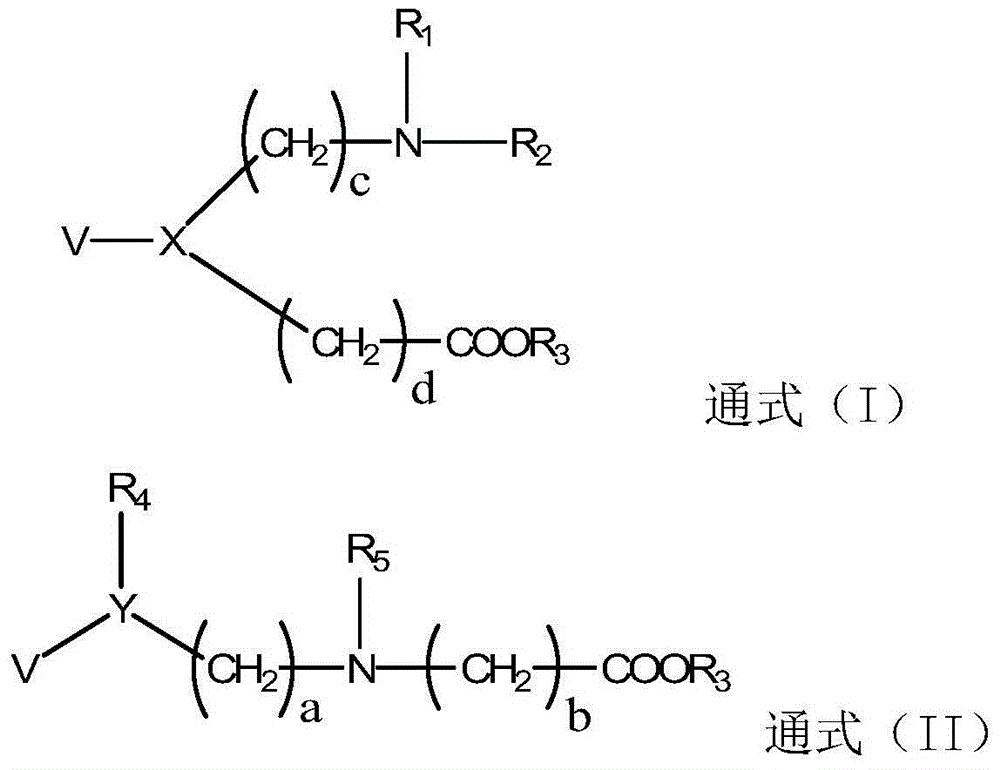
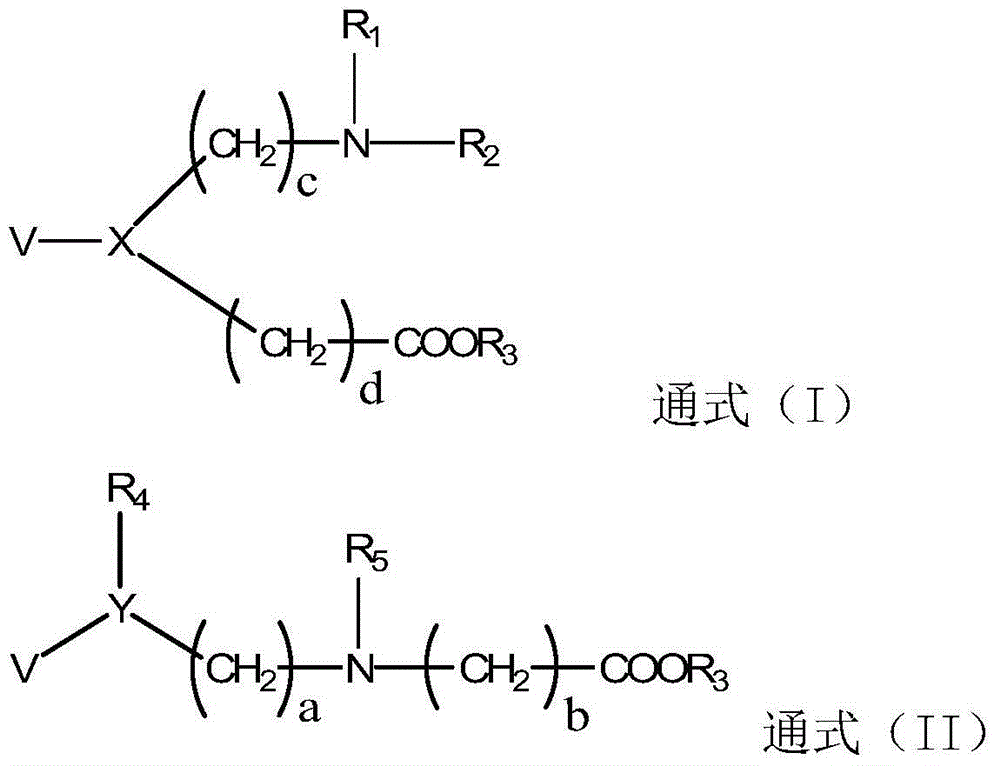
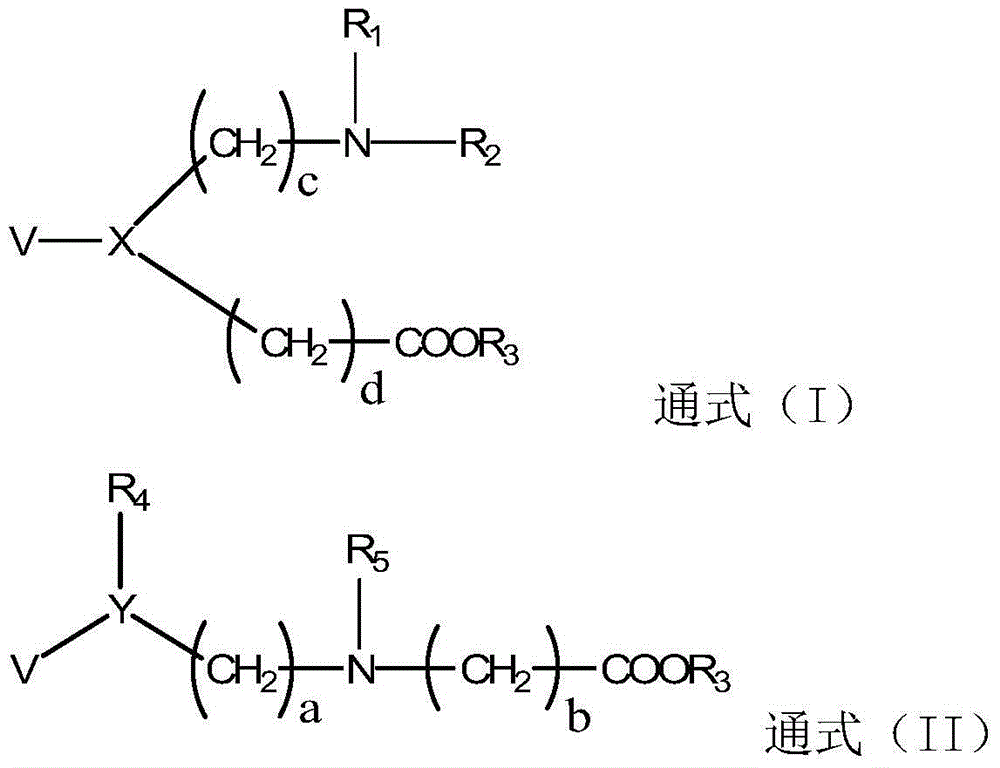
Betaine ester derivative, organic silicon material, preparation method and application thereof
A betaine ester and derivative technology, applied in the field of silicone materials, achieves excellent optical transparency, improves hydrophilicity, resists protein non-specific adsorption, and reduces infection effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Embodiment 1: Synthesis of betaine ester derivatives (see synthetic route 1)
[0093]a. Add 8.6g (0.1mol) of ethyl acrylate, 8.8g (0.1mol) of N,N'-dimethylethylenediamine and 100ml of solvent tetrahydrofuran into a single-necked flask, stir at 50°C for 24 hours, and then After distillation under mmHg, the components at 46-48°C were collected to obtain compound 3 with a yield of 85%.
[0094] b. Add 10.54g (0.06mol) of compound 3 obtained in the previous step, 6.68g (0.066mol) of triethylamine and 100ml of solvent tetrahydrofuran into a 250ml three-necked flask, take 6.90g (0.066mol) of methacryloyl chloride and put it into a 25ml constant Pressure drop funnel, nitrogen protection, ice bath slowly dropwise for 1 hour, after 24 hours of reaction, remove triethylamine hydrochloride by suction filtration, rotary evaporation to remove most of the solvent and residual triethylamine to obtain a light yellow liquid, the light yellow liquid Dissolve the yellow liquid in 100ml o...
Embodiment 2
[0096] Embodiment 2: Synthesis of betaine ester derivatives (see synthetic route 2)
[0097] a. Add 16.7g (0.1mol) methyl bromopropionate, 8.8g (0.1mol) N, N'-dimethylethylenediamine and 100ml solvent tetrahydrofuran into a single-necked flask, and stir at 50°C for 6 hours, Remove triethylamine hydrochloride by suction filtration, distill at 0.003mmHg, collect components at 46-48°C to obtain compound 3 with a yield of 70.5%.
[0098] b. Same as step b of Example 1, to obtain compound 5 (ie, betaine ester derivative of methacrylic acid), with a yield of 95.8%.
[0099]
Embodiment 3
[0100] Embodiment 3: Synthesis of betaine ester derivatives (see synthetic route 3)
[0101] a. Add 8.6g (0.1mol) of ethyl acrylate, 8.8g (0.1mol) of N,N-dimethylethylenediamine and 100ml of solvent tetrahydrofuran into a single-necked flask, stir at 50°C for 24 hours, and then dissolve at 0.003mmHg Under distillation, compound 7 was obtained with a yield of 88%.
[0102] b. Add 11.3g (0.06mol) of compound 3 obtained in the previous step, 6.68g (0.066mol) of triethylamine and 100ml of solvent tetrahydrofuran into a 250ml three-necked flask, take 6.90g (0.066mol) of methacryloyl chloride and put it into a 25ml constant Pressure drop funnel, nitrogen protection, ice bath slowly dropwise for 1 hour, after 24 hours of reaction, remove triethylamine hydrochloride by suction filtration, rotary evaporation to remove most of the solvent and residual triethylamine to obtain a light yellow liquid, the light yellow liquid Dissolve the yellow liquid in 100ml of dichloromethane, wash with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| contact angle | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com