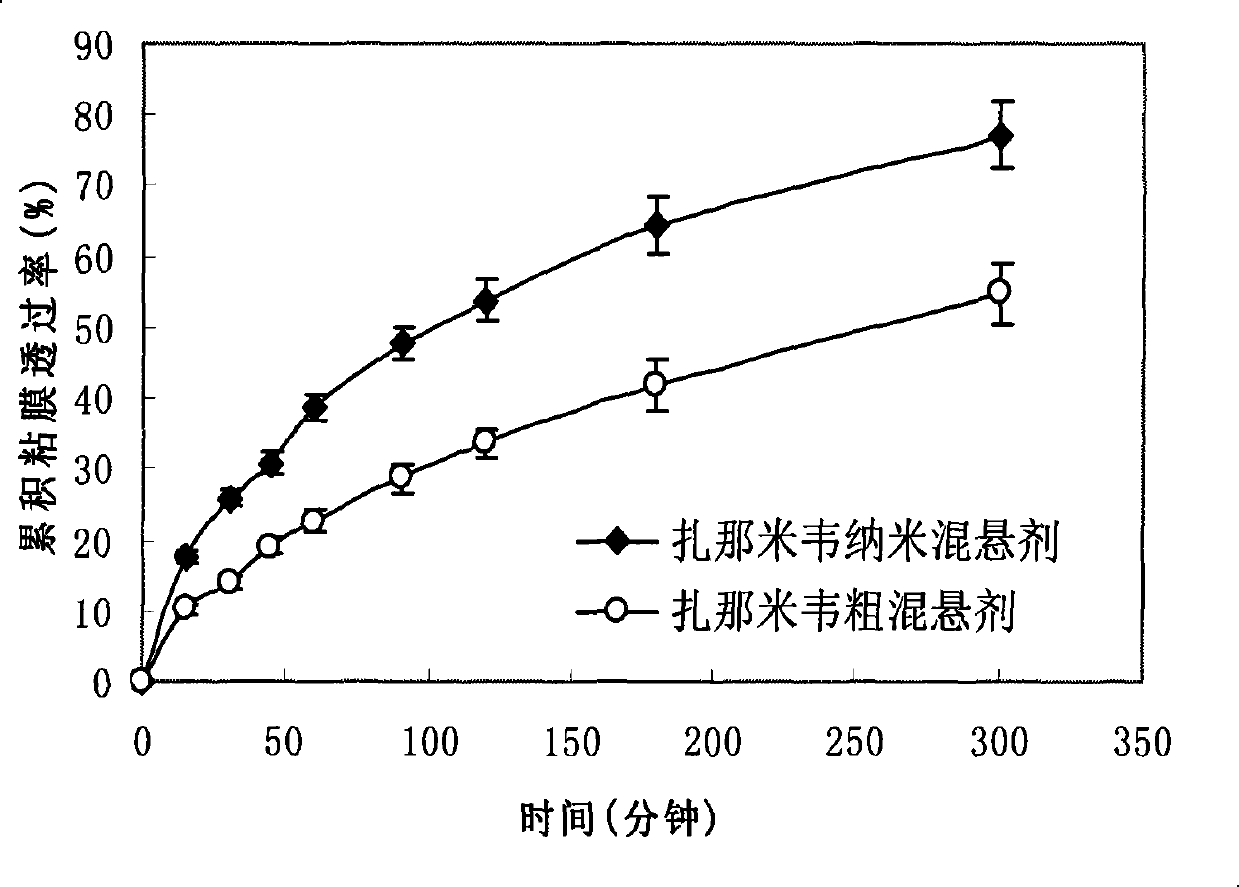
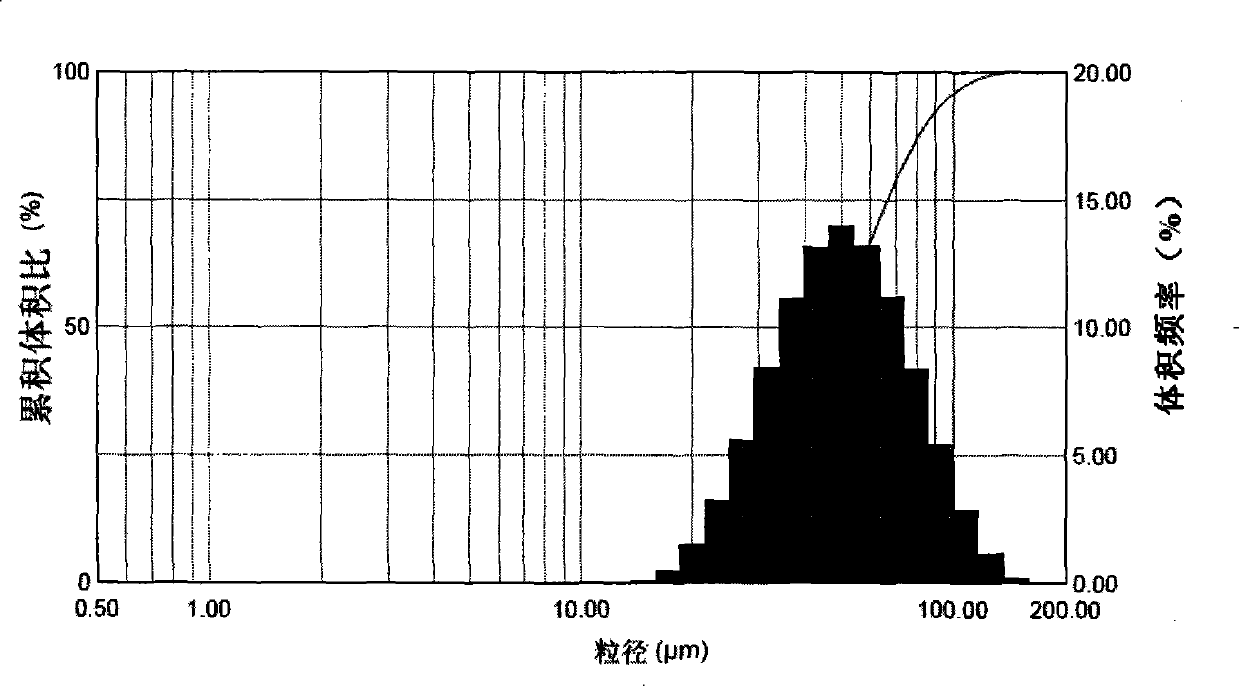
Zanamivir nasal nanometer suspension and preparation method thereof
A nano-suspension, zanamivir technology, applied in non-active ingredients medical preparations, antiviral agents, pharmaceutical formulations, etc., can solve the problem of oral bioavailability of only 2%, fast renal clearance, and bioavailability. It can improve the bioavailability of the drug, prolong the residence time, and make the preparation process simple.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] prescription:
[0052] Zanamivir 10%
[0053] Poloxamer 188 5%
[0054] Polyvinylpyrrolidone K-12 5%
[0055] Glycerin 2.6%
[0056] Appropriate amount of acetic acid, adjust the pH value to 4.0
[0057] An appropriate amount of trishydroxymethylaminomethane (30%), adjust the pH value to 6.0
[0058] water balance
[0059] Preparation:
[0060] The zanamivir bulk drug was jet milled (particle size 2 μm). Prepare an aqueous acetic acid solution with a pH value of 4.0, adjust the pH value to 6.0 with 30% aqueous trishydroxymethylaminomethane solution, add the remaining auxiliary materials in the prescription under magnetic stirring, dissolve, and finally make the zanamivir bulk drug under magnetic stirring The powder is added to the above solution to fully dissolve and disperse to prepare a Zanamivir coarse suspension.
[0061] Transfer the Zanamivir coarse suspension to a high-pressure homogenizer, homogenize at a low pressure of 350 bar for 2 weeks, raise the pr...
Embodiment 2
[0064] prescription:
[0065] Zanamivir 20%
[0066] Cremophor EL 12%
[0067] Hypromellose E5 10%
[0068] Propylene Glycol 2.6%
[0069] Chlorobutanol 0.5%
[0070] Appropriate amount of acetic acid, adjust the pH value to 4.0
[0071] Appropriate amount of 1M sodium hydroxide, adjust the pH value to 6.0
[0072] water balance
[0073] Preparation:
[0074] Zanamivir crude drug was pulverized with a ball mill (particle size: 10 μm). Prepare an aqueous acetic acid solution with a pH value of 4.0, add the prescribed amount of chlorobutanol, adjust the pH value to 6.0 with 1M sodium hydroxide aqueous solution after dissolving, add the remaining auxiliary materials in the prescription under stirring, dissolve, and finally dissolve under magnetic stirring Zanamivir crude drug powder is added to the above solution to fully dissolve and disperse to prepare a Zanamivir coarse suspension.
[0075] Transfer the Zanamivir coarse suspension to a high-pressure homogenizer, homog...
Embodiment 3
[0078] prescription:
[0079] Zanamivir 30%
[0080] Sodium Lauryl Sulfate 20%
[0081] Polyvinylpyrrolidone K30 10%
[0082] Mannitol 2.0%
[0083] Chlorobutanol 0.6%
[0084] Proper amount of hydrochloric acid, adjust the pH value to 4.0
[0085] An appropriate amount of Tris (30%), adjust the pH value to 6.0
[0086] water balance
[0087] Preparation:
[0088] Zanamivir crude drug was pulverized by mechanical grinding method (particle size 14 μm). Prepare an aqueous solution of hydrochloric acid with a pH value of 4.0, add the prescribed amount of chlorobutanol, adjust the pH value to 6.0 with 30% aqueous solution of trishydroxymethylaminomethane after dissolving, add the remaining auxiliary materials in the prescription under magnetic stirring, and dissolve , and finally, under magnetic stirring, the zanamivir bulk drug powder is added to the above solution to fully dissolve and disperse to obtain a zanamivir coarse suspension.
[0089] Transfer the Zanamivir coa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com